Meat alternatives made from soybeans and the like are merely substitutes for meat, but cultured meat, which is produced from the cultured cells of animals such as cows and pigs, is animal-derived protein and therefore “real” meat. While there are a number of production methods, particular attention is focusing on research aimed at mass production by means of regenerative medicine used to construct muscle and organ tissue from cells, with microorganisms serving as a source of nutrients for the cells. The goal is to produce something that genuinely feels like meat. One day, eating a tasty cultured meat steak will seem perfectly commonplace!
Special Feature 1 – Microorganisms and Sustainability Pursuing the “real” steak! Applying regenerative medicine to produce cultured meat
composition by Toshiko Mogi
The category of artificially produced meat includes both meat alternatives derived from soybeans and other plants, and cultured meat, which is grown from the cultured cells of livestock such as cows and pigs. My research focuses on the latter.
The concept of cultured meat is not a new one. While I only discovered it after starting to conduct research, Britain’s Winston Churchill discussed the idea in a 1931 essay, in which he wrote, “We shall escape the absurdity of growing a whole chicken in order to eat the breast or wing, by growing these parts separately under a suitable medium.” In Japan, artificial meat appears in Osamu Tezuka’s manga The Jungle Emperor.
Previously, that notion seemed like something out of a dream. That dream became reality in 2013. Professor Mark Post of Maastricht University in the Netherlands presented the world’s first lab-grown hamburger made from cultured cow cells. He developed it using a technique called tissue engineering. However, as it cost €250,000 (more than ¥30 million) to produce 140 g of cultured meat, commercialization was a long way off.
As technology evolved, various steps were taken to reduce the cost. A number of cultured meat startups have been established in various countries, including Japan, which are now competing with each other to develop commercial products. In Singapore, sales of cultured meat (chicken) were approved in 2020 for the first time anywhere across the globe. The cultured chicken reportedly went on sale at the beginning of 2021. I have also heard that cultured foie gras is going to be launched in Japan.
Producing meat without burdening the environment
There are three global reasons behind these moves (Figure 1). The first is food shortages due to an increase in meat demand resulting from population growth and rises in the standard of living (more diverse diets). There is concern about a protein crisis; that is to say, the risk of being unable to secure sufficient supply to meet the increase in demand.
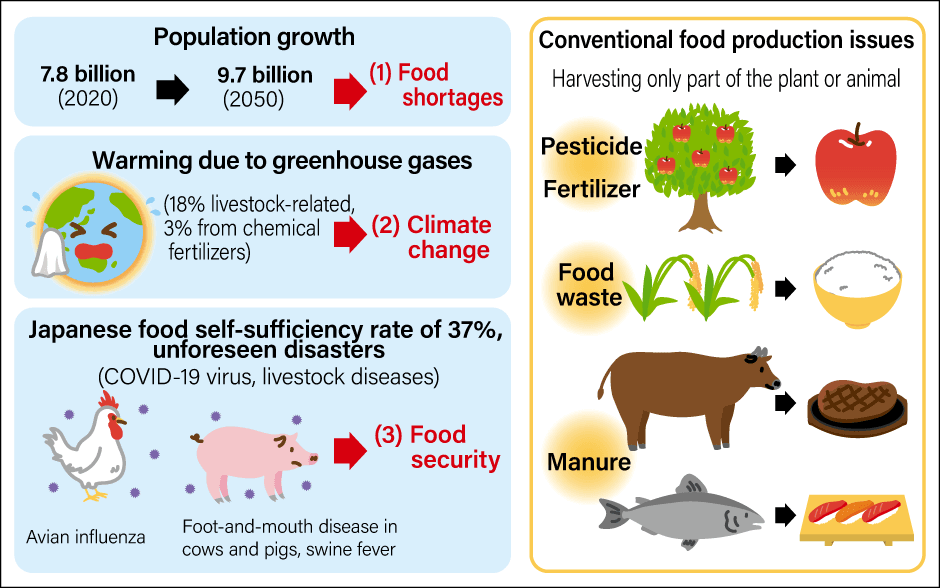
Figure 1. Factors behind the need to develop cultured meatA circular meat production system is needed to address food shortages due to population growth, the environmental burden associated with raising livestock, and food security issues in case of infectious diseases or other unforeseen disasters.(Illustration: Rina Mikami)
This is because increasing meat production is not easy. Meat production relies on having the land, water, grain feed, and animal waste treatment facilities essential to raising livestock. Some have pointed out that these can cause environmental destruction or climate change due to greenhouse gas emissions. In other words, as Churchill said, we need to move away from the conventional approach and adopt a sustainable form of meat production that does not impose a burden on the environment. This is the second reason.
The third is food security. We are currently facing an unforeseen disaster in the form of the COVID-19 pandemic. Livestock, too, suffer from viral infections, such as foot-and-mouth disease, which affects cows and pigs; swine fever, which affects pigs; and avian influenza, which affects chickens. We have experienced countless outbreaks of such viral infections, and each time they have been dealt with by culling animals and carrying out disinfection. If we become unable to raise food-producing animals due to such diseases, there will be meat shortages. Alternatively, should there be an outbreak of a zoonosis —— an infectious disease that has jumped from an animal to humans —— the health of both livestock and humans will be at risk. As we have already experienced during the COVID-19 pandemic, we do not know when or where unforeseen disasters might befall us, nor in what form. We must secure sufficient food supply even when the unexpected occurs.
So, how is cultured meat made? We are using tissue engineering methods in our efforts to develop a means of producing cultured meat.
“Meat” is the collective term for the muscle and fat that we consume. Muscle is tissue consisting of bundles of muscle fibers. Consequently, manufacturing cultured meat means making muscle tissue.
Let me put it simply (Figure 2). In the laboratory, we first collect muscle tissue from living livestock and culture cells called myoblasts. Single-nucleus cells from which muscle fibers are derived, the myoblasts divide and fuse to each other, ultimately becoming muscle fibers once mature. However, in this state they are still in paste form. They are then arranged into firm, chewy muscle tissue and appropriate amounts of fat and other substances are added before the product is shaped to create the finished cultured meat.
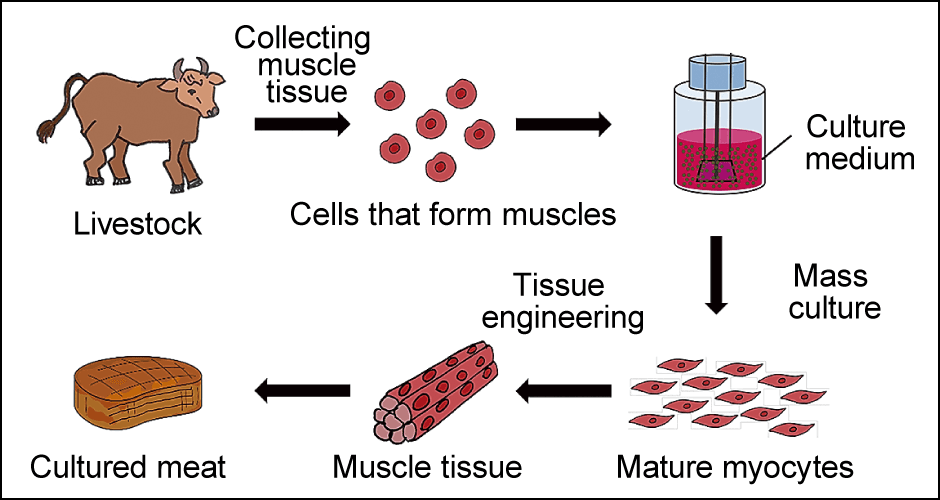
Figure 2. The process of making cultured meatCells that form muscles (myoblasts) collected from livestock are cultured using a culture medium containing various nutrients and the resultant cells are turned into tissue to create meat.
There are a number of challenges to overcome in order to commercialize cultured meat. The three key issues are the culture medium, the formation of a 3D structure, and tastiness.
Building a circular cell culture system using algae
Cell culture medium contains nutrients such as amino acids and glucose, which are derived from grain. While research uses only a small quantity of cell culture medium, a vast amount of culture medium will be needed once cultured meat begins to account for a significant proportion of meat production. This is an issue that needs to be resolved, from the perspectives of both cost and the environmental burden. Having become aware of this problem as my research progressed, I have begun exploring ways of building a circular cell culture system using algae. I would like to come back to this topic at the end.
The biggest difficulty of them all is the process for building muscle tissue. A number of methods can be used, such as seeding the cells in a porous, three-dimensional block made beforehand from biodegradable polymer materials or mixing the cells with collagen and the like before pouring the mixture into a mold. Other methods include lining the individual cells up three-dimensionally with a small amount of gel using a 3D printer or piling up a mass of cells on a device consisting of a metal plate with needles or a metal mesh and then withdrawing the device once the tissue has formed. We are producing cultured meat using the method described below, which involves layering cell sheets.
Finally, there is the challenge posed by tastiness —— that is to say, texture and flavor. Some cultured meats are mixed with hard substances or with fat and collagen derived from livestock in order to produce the required texture, but this deviates from the original purpose of avoiding the sacrifice of animals. We expect to be able to solve this problem and achieve the desired texture by tempering or layering the cells. Companies that manufacture and sell food are currently devising ways of tailoring the flavor to the preferences of end users. However, as it is possible to design unique meats, it would be wise to give consideration to ensuring that they are healthy and highly nutritious. In my personal opinion, if we could add the requisite nutrients at the mince stage and make it taste good, cultured meat could become a nutritious meal that is easy to eat for elderly people whose ability to chew is impaired.
So far, we have succeeded in producing slices of cultured ham about 1 mm thick by layering sheets of bovine (cow) myoblasts (Figure 3). Unfortunately, we do not know how it tastes. This is because cultured meat was produced using research regents in university laboratories.

Figure 3. Cultured ham made with cell sheetsCultured ham created in the current phase of research. It is a little bigger than the cherry tomato beside it on the plate.
(Produced through collaborative research between Tokyo Women’s Medical University and Waseda University)
Since 1999, I have been engaged in regenerative medicine research aimed at making hearts to treat heart failure. One area of research in regenerative medicine focuses on how to build tissue and organs from cells. The reason why I and many other researchers in the field of regenerative medicine are working on cultured meat is that both use tissue engineering to produce tissue from cells.
There are a number of ways to make tissue from cells. Cultured meat is composed of cells encased in an extracellular matrix providing structural support. In the main method employed to date, biodegradable polymer scaffolds have been used as an alternative to the extracellular matrix. However, these approaches still pose issues of food safety and quality that need to be resolved, as some of the scaffold may remain in the product and the extracellular matrix should be replaced by connective tissues of the principal component. Accordingly, rather than using a scaffold, our research team is layering cell sheets that we have produced (Figure 4).
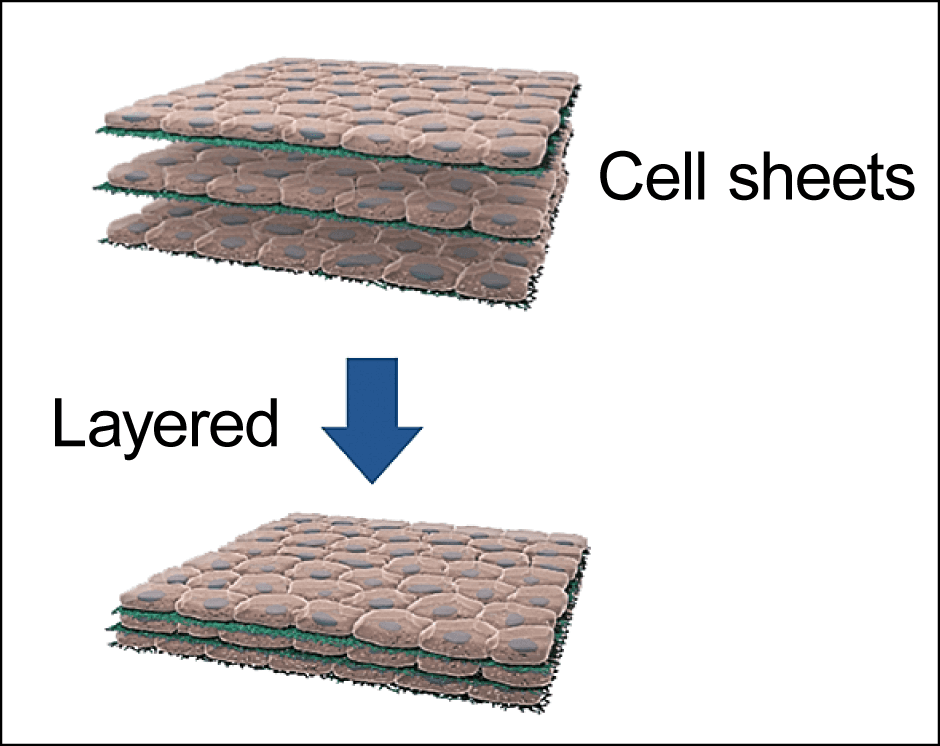
Figure 4. The cell sheet techniqueAlgae and cell sheets removed from temperature-responsive culture dishes are layered alternately, building up a 3D cell structure.
Cell sheets developed via regenerative medicine research
If cells are cultured as a sheet in an ordinary Petri dish, proteins cause the cells to bind to each other and stick to the dish. Trying to remove the cell sheet by dissolving the proteins results in the cells separating again, breaking up the sheet. A special kind of Petri dish called a temperature-responsive culture dish has been developed to resolve this problem and using these special culture dishes enables us to remove the cell sheet from the dish simply by changing its temperature.
Cell sheets were originally developed in the course of regenerative medicine research. They are already used for a variety of applications in clinical trials and clinical settings. Covered by health insurance since January 2016, sheets of this nature include Terumo Corporation’s HeartSheet, which is used to treat serious heart failure. The treatment is designed for patients suffering from severe heart failure, whose heart muscle has become thin and dilated, weakening its ability to pump blood around the body. It involves producing cell sheets made from muscle cells (skeletal myoblasts) taken from the patient’s own thigh and attaching the sheets to the patient’s heart. Due to the effects of cytokines, which activate cells in the surrounding heart tissue, the muscle regains its ability to pump blood around the body.
Thus, espousing the unique concept of cell sheet engineering, which involves producing and transplanting single or multi layered sheets of cells, we in the field of regenerative medicine have been engaged in research and development. We are also moving forward with academic-industrial collaborative research into cultured meat that taps into the outcomes of our work. While we are still only at the level of thin slices of ham at the moment, we aim to build up the layers of our cell sheets gradually, so that we ultimately form 3D tissue that genuinely feels like meat —— in other words, building a steak.
I mentioned earlier that one of the issues we need to resolve in producing cultured meat is the fact that the culture medium is derived from grain. We need to switch to a sustainable culture medium. Accordingly, we began to focus on algae.
Algae are microorganisms, and microorganisms have already been put to such uses as degrading meat proteins to draw out umami peptides. So where did this idea of reversing this process come from? In fact, it originated in regenerative medicine research.
Even if cell sheets are layered, oxygen and nutrients cannot be supplied to the cells without a network of blood vessels. In this situation, ammonia and other toxic metabolites accumulate, causing cellular necrosis. However, we succeeded in producing cardiac muscle tissue with a thick 3D structure by layering algae and cell sheets on top of each other like a sandwich and culturing them together. This symbiotic recycling system combining mammalian cells and algae gave us the idea for using algae to produce cultured meat as well.
When exposed to light, algae produce nutrients in the form of polysaccharide and proteins from inorganic substances such as CO2 and nitrogen sources. Accordingly, we decided to extract those nutrients from algae instead of grain and decompose them to create a culture medium. It is already possible to extract from several types of algae the basic nutrients required to culture animal cells, namely glucose, amino acids, and vitamins. Our experiments confirmed that liquid extracts of these algae serve as an alternative to the nutrients in conventional culture medium. We also confirmed that the algae purify the culture medium by using the ammonia emitted by the animal cells to produce amino acids. Based on these findings, we hope to build a system for the circular culture of animal cells and algae with photosynthetic capabilities, in which, as long as light energy is available, the algae provide the animal cells with nutrients and vitamins, and the animal cells use those to produce the ammonia and other substances needed by the algae; in other words, a circular culture system in which each element gives the other what it needs. We are currently engaged in research and development as part of a Moonshot Agriculture, Forestry and Fisheries Research and Development Program entitled “Bio-economical food production system using circular cell culture of algae and animal cells” (Figure 5).
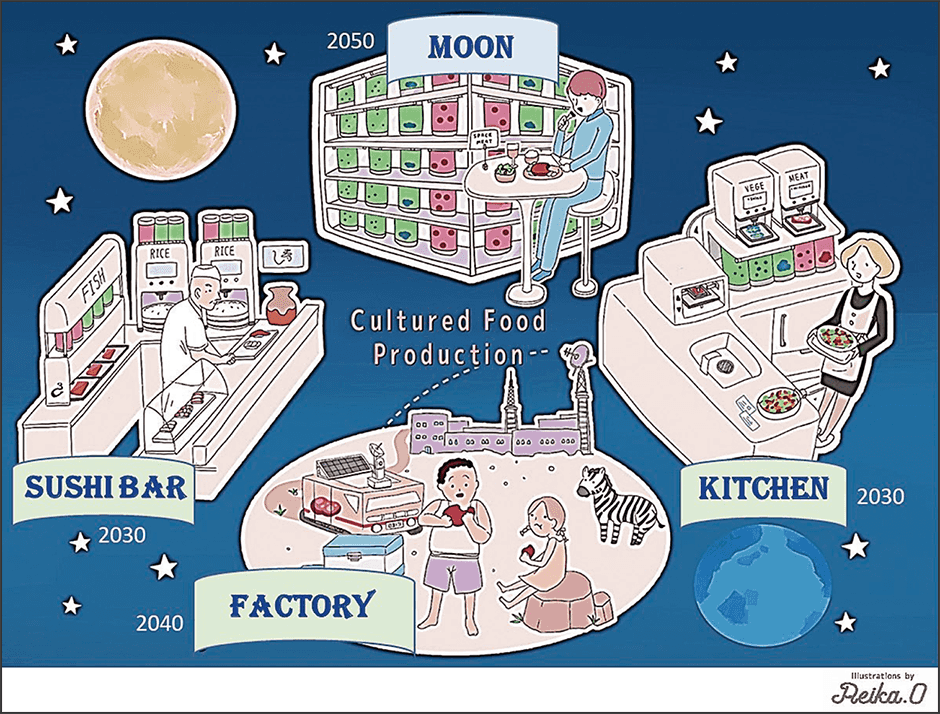
Figure 5. Vision for a future circular system of cultured food (meat) productionThis vision for the future depicted by the research team is the target they aim to achieve on Earth and in space in the near future. This circular system ensures food security without imposing a burden on the global environment.
A program aiming to produce food in space
If this circular system can be established, it would be possible to transform our meat production system based on raising grain-fed livestock into a cultured meat production system in which algae cultured with the aid of light energy are used as a source of nutrition for culturing myoblasts, which are then turned into tissue. This also shows that meat production will be possible even in closed environments such as space and Antarctic research stations. An organization called the Space Foodsphere Association, in which a number of companies and universities including our research team are involved, has already launched a program aimed at producing food in space and is working toward the goal of producing cultured meat on the moon in the latter half of the 2030s.
As it happens, around the time I was moving into regenerative medicine from clinical practice as a cardiovascular physician, I harbored dreams of becoming an astronaut after seeing an astronaut recruitment notice. Perhaps because of this, I have been interested in space ever since. Now that I can be involved with space through my research, creating a system for sustainable meat production in space is both my research theme and my dream.
Both Churchill’s prediction/proposal and Osamu Tezuka’s depiction of artificial meat in The Jungle Emperor seemed like the stuff of dreams at the time. However, scientific progress has already made a number of dreams come true. I am confident my own dream will come true. I hope that research into cultured meat will also help to realize the long-cherished dream in regenerative medicine of making new hearts.

















