When it comes to the gut–brain axis —— the connections via which the brain and the intestines influence each other —— it is not all that easy to create a good intestinal environment. However, the existence of factors controlling the intestinal microbiota and keeping them in an appropriate state has been revealed, and scientists have discovered that Paneth cells in the small intestine are related to those factors. Shedding light on the mechanisms will enable a completely new approach to be taken to mental and physical health.
Special Feature 1 – Brain, Body, and Mind Factors and cells controlling the gut–brain axis
composition by Rie Iizuka
The small intestine has recesses called crypts, the bases of which reside cells that produce large granules (Figure 1). These are called Paneth cells and are found only in the small intestine. They play a part in innate enteric immunity, secreting granules containing antimicrobial peptides called α‐defensins, which have powerful bactericidal properties, when pathogens invade the intestinal tract.
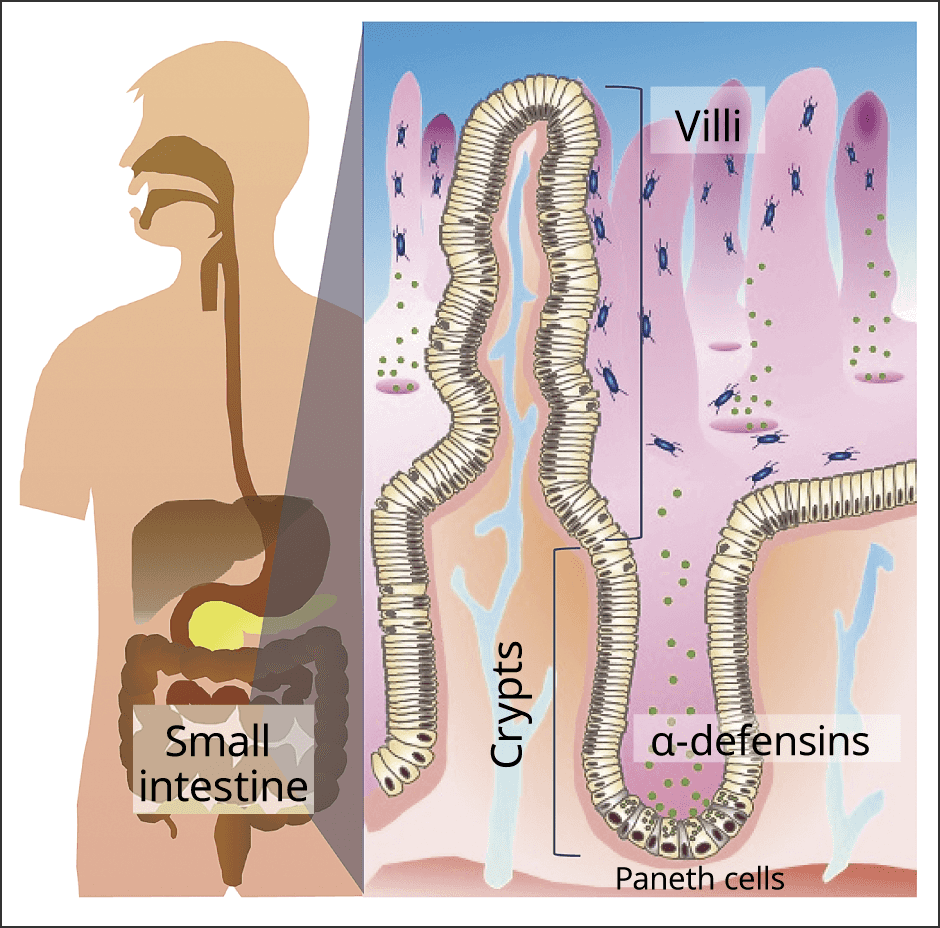
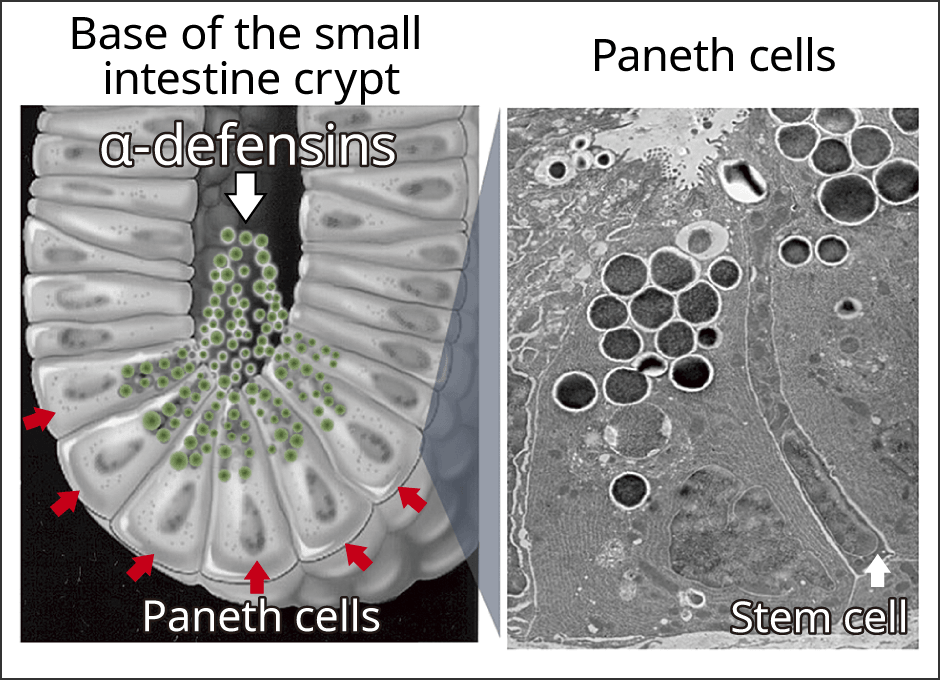
Figure 1. Paneth cellsFound in the small intestine, Paneth cells are the front line of our innate immunity.
Scientists know that the intestinal microbiota is disrupted when people suffer from depression, but the specific mechanism involved has not been discovered. We propound that the effects of α‐defensins might be involved in this process.
Factors controlling the intestinal microbiota
In our research to date, when we looked at the bactericidal activity of α‐defensins in regard to major enteric bacteria, including normal microbiota, we discovered that α‐defensins eliminate not only harmful bacteria (pathogens) but also opportunistic bacteria that can be harmful when allowed to overproliferate. However, Bifidobacterium, Lactobacillus, and other beneficial bacteria required to maintain health are not destroyed. From these, we can say that α‐defensins choose bacteria, eliminating pathogens and symbiosis with the intestinal bacteria beneficial to the host (Figure 2).
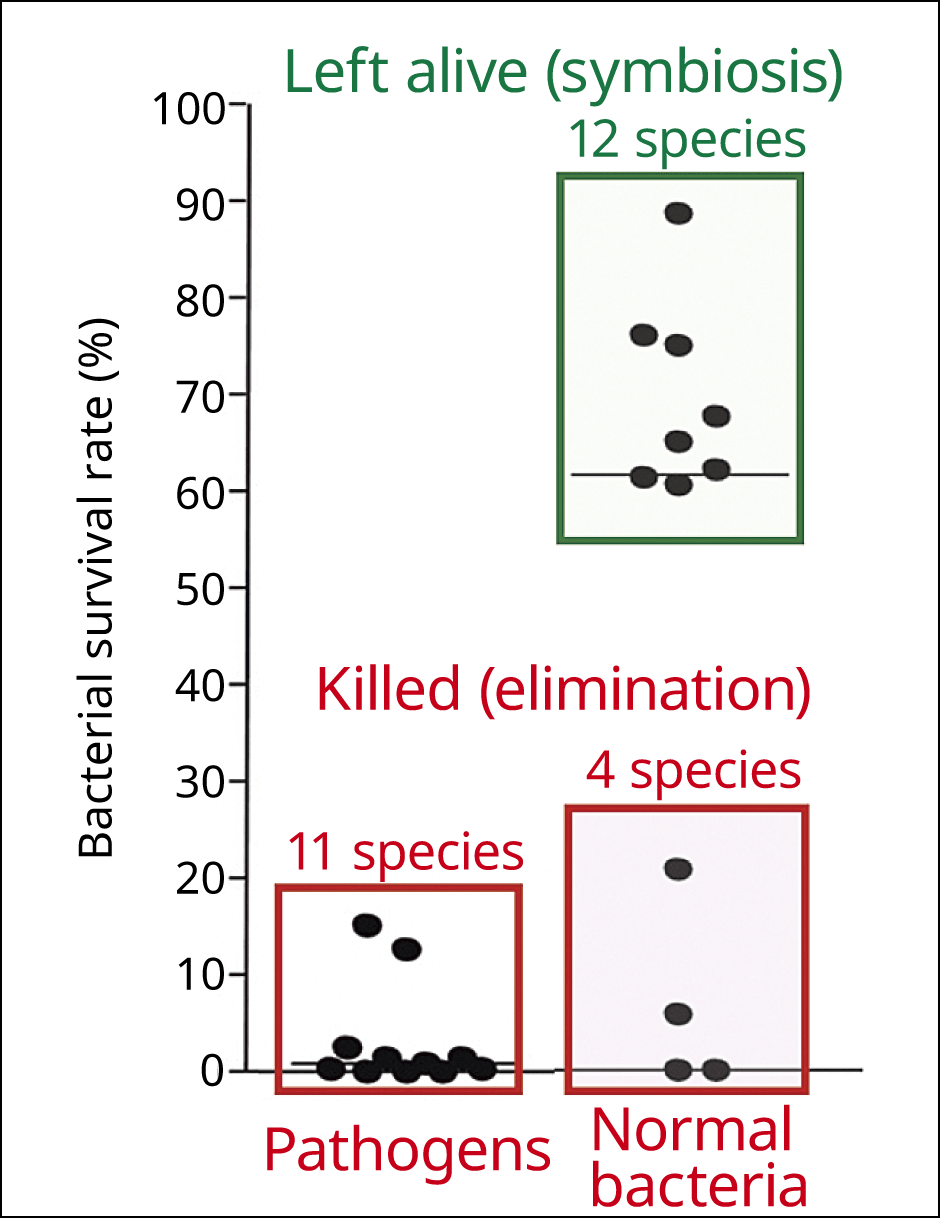
Figure 2. α‐defensins and the gut microbiotaIn normal mice (wild type) in which α‐defensins are being produced normally, bacteria harmful to the host (enterococci) and opportunistic bacteria that could be harmful if allowed to overproliferate (Bacteroides, etc.) are eliminated, whereas bacteria with a positive effect (Bifidobacterium and Lactobacillus) are not.
Hence, we assume that α‐defensins are the factor controlling the composition of the intestinal microbiota to keep it in a state appropriate to the host, and that they might therefore be related to various diseases and conditions such as Crohn’s disease and depression.
When we then investigated the small intestines of mice presenting depression-like symptoms due to psychological stress, we found a decline in both the number of granule-secreting Paneth cells and the quantity of α‐defensins. In addition, the composition of the intestinal microbiota clearly differed from that in healthy mice. The intestinal microbiota produces metabolites mainly in the form of short-chain fatty acids such as acetic acid and butyric acid, and the precursor to serotonin. Serotonin is a neurotransmitter which is involved in mental stability. It is thought that a shortage or even an oversupply of these metabolites affects the brain via blood vessels or nerves. In the case of metabolites of our mouse models of depression, we found a decrease in glutamic acid and uracil, among others, which are known to be involved in brain function. When we then orally administered α‐defensins to these mice, we found the composition of their intestinal microbiota returned to something close to a state found in healthy mice, with the glutamic acid, uracil, and other metabolites restored to normal levels (Figure 3).
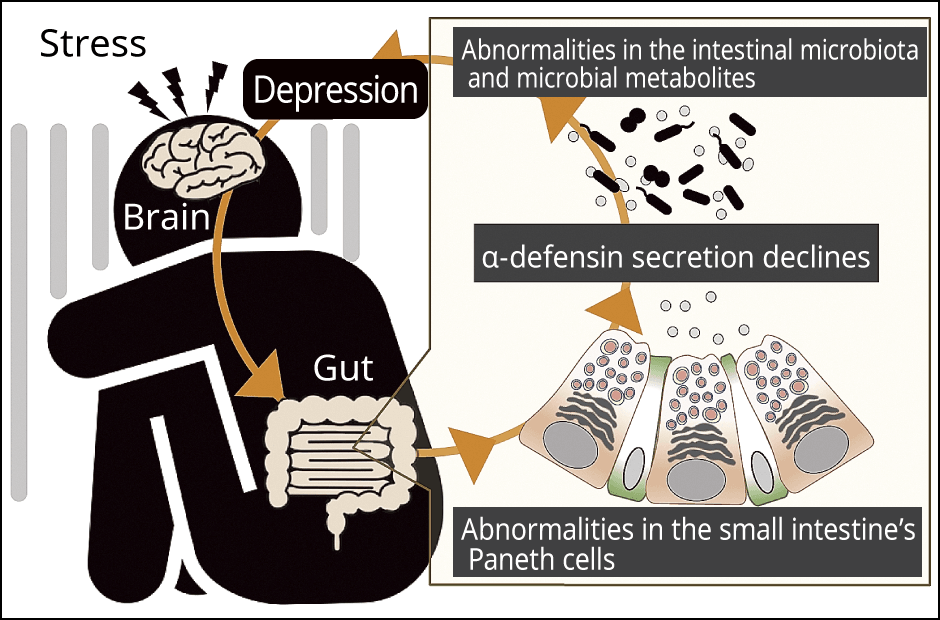
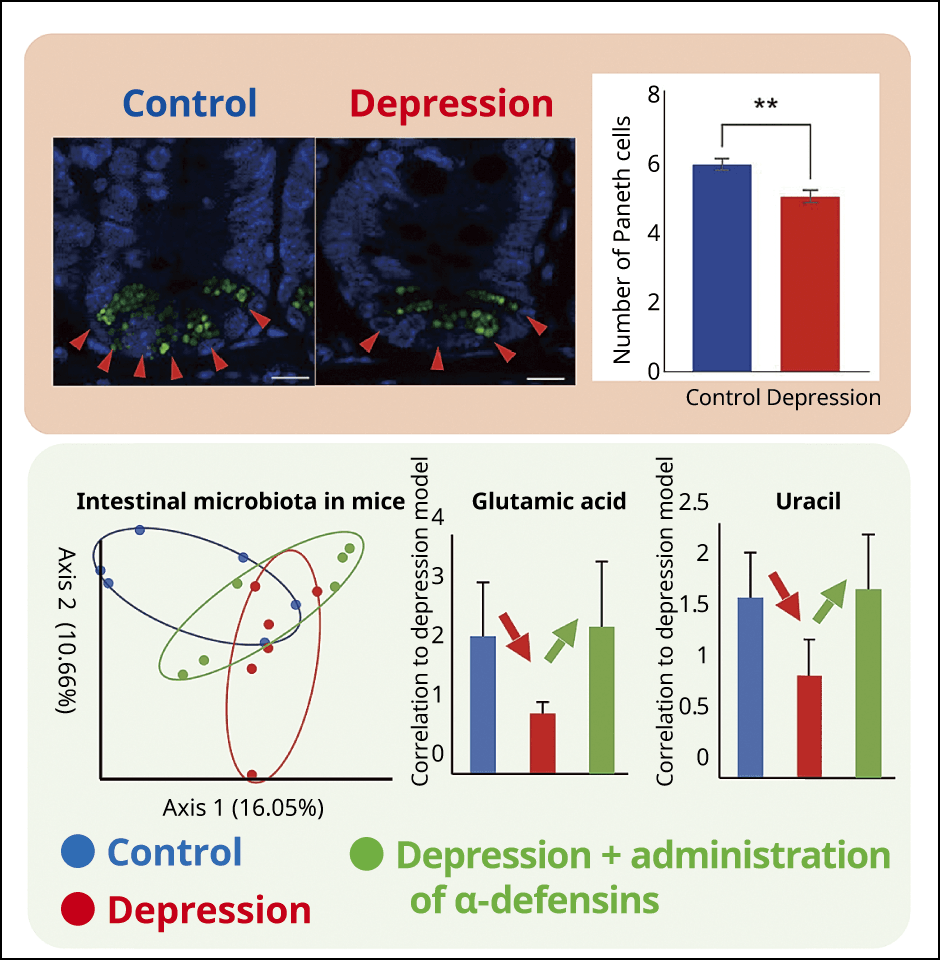
Figure 3. The relationship between depression, a decrease in α‐defensins, and the intestinal microbiotaThe number of Paneth cells decreased in the group with depression and there was a decline in glutamic acid and uracil, which are intestinal microbial metabolites. The quantity of metabolites increased to the previous normal level when α‐defensins were administered orally. The bottom left diagram shows the features of the intestinal microbiota in each of the mice (1 dot represents 1 mouse). The nearer the dots are, the greater the similarity in the features of the microbiota; dots with more spaced apart indicate different intestinal microbiota. From this, we can determine the diversity of the intestinal microbiota.
In our study, we could show that intestinal microbiota is disrupted when stress causes a decrease of Paneth cells and α‐defensins, and that the intestinal microbiota recovers when α‐defensins are administered orally. This means that, if used appropriately, α‐defensins could conceivably provide an approach to tackling depression.
The composition of the gut microbiota changes as we age. The risk of developing conditions such as obesity, diabetes, fatty liver, dementia, and depression increases with age and some reports have suggested that this might be due to changes in our intestinal microbiota. We therefore think that a decrease of α‐defensins might be a contributing factor.
Paneth cells also play a crucial role
In a community cohort study conducted in the Hokkaido town of Suttsu, we measured the quantity of α‐defensins in residents aged from 35 to 81 years old. We found that the quantity of α‐defensins in feces declines with age. This result suggests a correlation between the deterioration in the intestinal environment due to a decrease of α‐defensins and the increasing risk of disease as we age.
The decrease in α‐defensins is linked to declining intestinal immune function. We believe that keeping α‐defensins at appropriate levels would inhibit the decline in various immune functions caused by aging, thereby moderating the risk of disease.
We are currently exploring ways of controlling appropriate levels of α‐defensins through diet.
Paneth cells that secrete α‐defensins themselves are closely related to our health. They are abundant in younger age, and when we look at the small intestines in mouse models of Crohn’s disease, which causes chronic enterocolitis in younger age, we can see that the villi and crypts are quite swollen compared with those of healthy mice. In Paneth cells of healthy mice, the granules are visible and uniform, with the endoplasmic reticula that synthesize proteins arranged in an orderly manner. In the Crohn’s disease models, however, one can see that the granules inside the Paneth cells are shrunken, and the endoplasmic reticula have deformed to a wavy structure. Thus, the endoplasmic reticula cannot synthesize proteins properly and a large quantity of α‐defensins with an abnormal, thread-like structure (reduced-form α‐defensins) is produced. These reduced-form α‐defensins also eliminate all the bacteria beneficial to the host, reducing the diversity of the intestinal microbiota. As the quantity of reduced-form α‐defensins increases, the Crohn’s disease score (severity of the condition) also rises. So, not only the quantity, but also the quality of α‐defensins is important in maintaining the health of the host. Thus, we can see that Paneth cells must function normally to secrete intact α‐defensins.
Paneth cells are located adjacent to stem cells. Epithelial cells of the intestinal tract are replaced by new cells in a cycle of just three days, and this metabolism is induced by the stem cells. Previous studies have found that the Paneth cells send signals to the stem cells to adjust cell metabolism by creating stem cell niche. The only organ that regenerates cells in such a short period is the intestine. The intestine is the organ that obtains nutrients from foods, and younger epithelial cells are able to absorb nutrients more efficiently. At the same time, as death can result if pathogens enter along with the foods, our intestine needs to be able to attack them immediately. Thus, it is only reasonable that antimicrobial peptide secreting Paneth cells should be located in this organ. Paneth cells play a crucial role in the human process of eating to receive nourishment and live a healthy life.
An indicator for assessing the intestinal environment
We believe it is possible to take a completely new approach to mental and physical health, now that light has been shed on the functions of Paneth cells and α‐defensins. Since the knowledge that a good intestinal environment helps to maintain good mental and physical health has become widespread, numerous studies focused on which bacteria are good for us have been conducted. Due to the mass controvertial information, it is difficult for people to find the right answer regarding intestinal microbiota suitable for themselves.
Leaving aside those that are clearly good for us such as Bifidobacterium and Lactobacillus, there are cases in which, a particular bacterium might be harmful for us if allowed to overproliferate, but its presence might be what maintains the balance of intestinal microbiota in some people. Eating habits and living environments vary from one country or region to another, and it is apparent that the patterns of intestinal microbiota composition also vary. The intestinal microbiota differs from one individual to another. The question of how the network of innumerable bacteria present in the intestine affects the host is very complex, so it is difficult to define a single “good intestinal environment.”
I believe that α‐defensins could serve as an indicator for assessing the intestinal environment.
The main area of research in the intestinal microbiota has been probiotics, which is principally focused on bacteria, but our team focuses on the host and is working on research aimed at how to ensure adequate secretion of α‐defensins.
The symbiotic bacteria in the intestine are our partners, and they could be even regarded as a part of our body. Earth is home to a variety of bacteria, and the symbiotic bacteria live in the human body. The human body does not accept all bacteria, with as many as 70% or so being eliminated, and over a long period of time, we have managed to establish symbiosis with those bacteria that we can accommodate and with which we can maintain a win-win relationship.
We believe that humans themselves, as their hosts, are capable of controlling these symbiotic bacteria. We can choose what we eat and eliminate harmful bacteria from the body using antibiotics.
If α‐defensins are involved in making choices beneficial to the host regarding elimination and symbiosis, it means that the optimal intestinal microbiota for the host is created when Paneth cells and their α‐defensins are functioning properly. The scientific importance of probiotics is obvious. However, we know what constitutes of our own body play a role in healthy intestinal environment. Therefore, our team are pursuing research focused not only on the intake of the bacteria we lack from external sources but also using diet to regulate the body’s functions via Paneth cells.
Using medium to grow tissue called enteroids —— the same tissue found in the crypts of the small intestine —— in a culture dish and observe the secretion of granules by Paneth cells when nutrients and intestinal microbial metabolites are added, we can see that Paneth cells secrete granules in response to certain short-chain fatty acids and amino acids. We have also confirmed that Paneth cells express nutrient receptors and transporters. We hope that, if we can use nutrients or foods to stimulate Paneth cells and induce the secretion of α‐defensins, it might be possible to use self-care to cultivate a body resistant to stress and thereby prevent depression and other diseases brought on by psychological factors. We believe that focusing on the activity of Paneth cells rather than tackling the microbiota directly is a new approach to regulating the intestinal microbiota.
Improving health through self-care focusing on intestinal microbiota
Intestinal microbiota affect homeostasis of the host, and Paneth cells also are affected by the intestinal microbiotat. Mice raised in an aseptic environment have underdeveloped Paneth cells. Diverse intestinal microbiota is required for Paneth cells to function fully.
In mice raised normally, the number of Paneth cells increases rapidly from the time immediately after weaning until reaching adulthood. After weaning, the intestinal microbiota becomes more diverse, and the period when the ideal composition of bacteria for the individual is established coincides with the period when the sharp rise in Paneth cell number occurs. Thus, Paneth cells also play an important role in the period determining the composition of our intestinal microbiota throughout our lives.
If this is the case, nutrition not only around the time of weaning but also from the time we are still in the mother’s womb might have some kinds of effects. As there is information to suggest that a pregnant mother’s being obese or underweight is correlated to the child’s future risk of disease, I am focusing on the development of Paneth cells during the fetal period and in infancy.
When investigating the quantity of α‐defensins in the intestine, we currently measure the quantity excreted in feces. We already established the human α‐defensin measurement method, so I believe that being able to test people’s α‐defensin levels as part of their medical examinations would make it possible to contribute to maintaining the health of people. In the near future, I would like to apply chromatography to facilitate measurement in people’s own homes. Once people can carry out tests to check conditions by themselves whether their intestinal microbiota is well-controlled and then improve their diet to suit their individual circumstances, they will be able to check for and prevent a variety of mental and physical illnesses. These include not only lifestyle-related diseases but also depression, other stress-related conditions, and disorders related to aging. With my research, I would like to enable people to take care of their health.