While scientists have long known that the different bacteria that live in our gut affect our health, the public has been much less aware of this fact. Over the past decade, however, advances in analytical technology have begun to reveal the specific mechanisms. For example, scientists have discovered that gut bacteria are involved in aspects previously thought to be strongly influenced by our genome, such as our body weight and physical constitution, as well as the efficacy of drugs. Research into postbiotics —— metabolites produced by gut bacteria —— and the symbiotic relationship between different species of bacteria is also reported to be progressing. At the moment, gut bacteria are attracting as much attention as our genes.
Special Feature 1 – Forefront Research into Gut Bacteria Revealing the specific mechanisms of health effects of gut bacteria
composition by Rie Iizuka
illustration by Koji Kominato
The study of gut bacteria has progressed dramatically over the past 10 years. While we have long known that gut bacteria affect human health in a variety of ways, advances in analytical technology have rapidly revealed the specific mechanisms. In a number of cases, aspects of our physical constitution that we thought were determined by our genes have actually been shown to be due to gut bacteria.
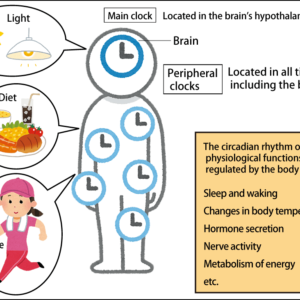
Gut bacteria affect various body conditions and medication effectiveness
The suggestion that gut bacteria could influence body weight, came from research on fecal microbiota transplantation. In some countries, fecal microbiota transplantation has been approved as a treatment for Clostridioides difficile infection. Although this treatment cures the infection, patients who receive fecal microbiota transplants from an obese person increase their body weight. In 2006, Professor Jeffrey Gordon at Washington University in St. Louis, USA, discovered that obese people have a high proportion of bacteria belonging to the phylum Firmicutes (now known as Bacillota).
Subsequent research then brought Akkermansia into the spotlight as a weight control bacterium. It was already known that Akkermansia promoted the growth of the intestinal mucosal layer, but further research revealed that this bacterium was also involved in curbing obesity. In Europe, pasteurized Akkermansia has been approved as a dietary bacterial supplement for weight control.
Gut bacteria are also known to affect the efficacy of drugs. This phenomenon has become widely known as a result of the drug levodopa, which is used to treat Parkinson’s disease. Levodopa is an excellent oral drug that has been used for more than 30 years, but there is considerable variability in its effects between individuals, and there have also been cases of it becoming less effective in the same person the longer they take it. Research has shown that two bacteria —— Enterococcus faecalis and Eggerthella lenta —— metabolize levodopa, preventing the body from absorbing its active ingredients. Accordingly, the effectiveness of levodopa was restored by giving patients a drug that inhibited its metabolism by these bacteria.
There are also reports that gut bacteria can even alter a person’s athletic ability. Marathon runners with outstanding times have been reported to have high levels of Veillonella in their gut bacteria. In addition, feeding Veillonella to mice increased their physical endurance. In other words, just one type of gut bacteria affects a person’s ability to exercise.
In collaboration with local governments and other organizations across Japan, we are collecting and analyzing data from a total of 10,000 people. The results have provided us with a number of very interesting discoveries about the gut bacteria of Japanese people.
One is individual differences in gut bacteria. It was previously thought that Bifidobacterium was abundant in the gut microbiota of Japanese people, accounting for about 10-15% of all gut bacteria. However, there was actually considerable variation between individuals in the amount of Bifidobacterium in their guts (Figure 1). In other words, it was not the case that all Japanese people had a large amount of Bifidobacterium, but rather that the figure was an average based on a situation in which a certain proportion of the population had exceptionally high levels, while others had hardly any.
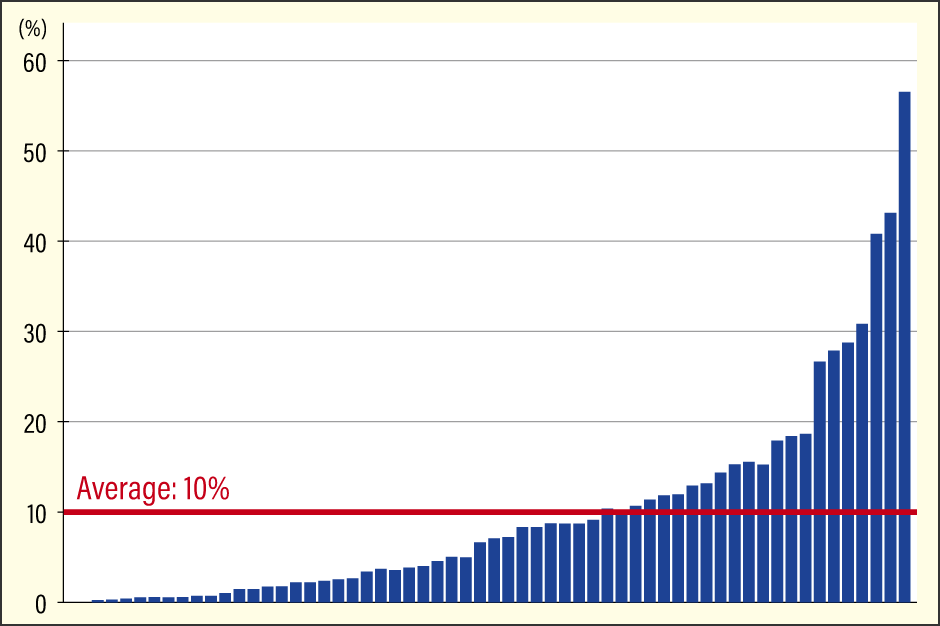
Figure 1. Bifidobacterium in the gut microbiota of Japanese peopleIn a survey of Japanese people, the average percentage of Bifidobacterium in the intestine was about 10%, but this is only an average, and Bifidobacterium do not make up 10% of the intestinal microbiota in all Japanese people. There was considerable individual variation, with some people having no Bifidobacterium at all and others having over 50% Bifidobacterium.
The second key finding relates to bacteria for weight control in Japanese people. Compared to people in other countries, the Japanese have a low obesity rate, so they tend to have a low BMI. However, we found Akkermansia —— identified in European studies as a weight-loss bacterium —— at levels of 1% or higher in less than 10% of Japanese people, showing that the Japanese have hardly any of this type of bacteria. This means that Akkermansia is not responsible for the slender physique of the Japanese.
Diabetes risk is lower in those with a higher level of Blautia
In a study of Japanese subjects, Blautia was identified as an alternative to Akkermansia for weight control. In other words, people with high levels of Blautia have a lower risk of obesity and type 2 diabetes. This is the third or fourth most common bacterium in the intestinal microbiota of the Japanese.
As factors involved in weight control, Blautia produced metabolites called ornithine, S-adenosylmethionine, and acetylcholine. These metabolites are known to promote metabolism and inhibit inflammation. In addition to producing these metabolites, Blautia also serves as bait for other beneficial bacteria. Blautia accumulates amylopectin, a type of resistant starch that is not digested in the small intestine and, like dietary fiber, feeds intestinal bacteria, thereby becoming food for other beneficial bacteria and improving the intestinal environment. Taken together, Japanese people are less likely to gain weight not only because of their genes, but also because of the influence of gut bacteria, one of which is thought to be the effect of Blautia.
Ornithine and other metabolites generated by bacteria are referred to as “postbiotics.” Typical examples include the short-chain fatty acids, such as acetic acid, butyric acid, and propionic acid. Postbiotics has become an important keyword in gut bacteria research. This is because postbiotics are both useful for human health and key players in the symbiosis of bacterial species.
When people hear that dietary fiber is metabolized by gut bacteria to produce short-chain fatty acids, they tend to assume that a particular species of gut bacteria breaks down the fiber and immediately produces useful short-chain fatty acids. In reality, however, it is not that simple.
In the intestine, dietary fiber and oligosaccharides are first broken down into sugars by amylolytic bacteria. Lactic acid bacteria and bifidobacteria then convert these sugars into lactic acid and acetic acid, which are used by other intestinal bacteria as materials to produce propionic acid and butyric acid. In this way, bacteria relay the food we eat, gradually changing its form to produce postbiotics that serve as batons in this relay (Figure 2).
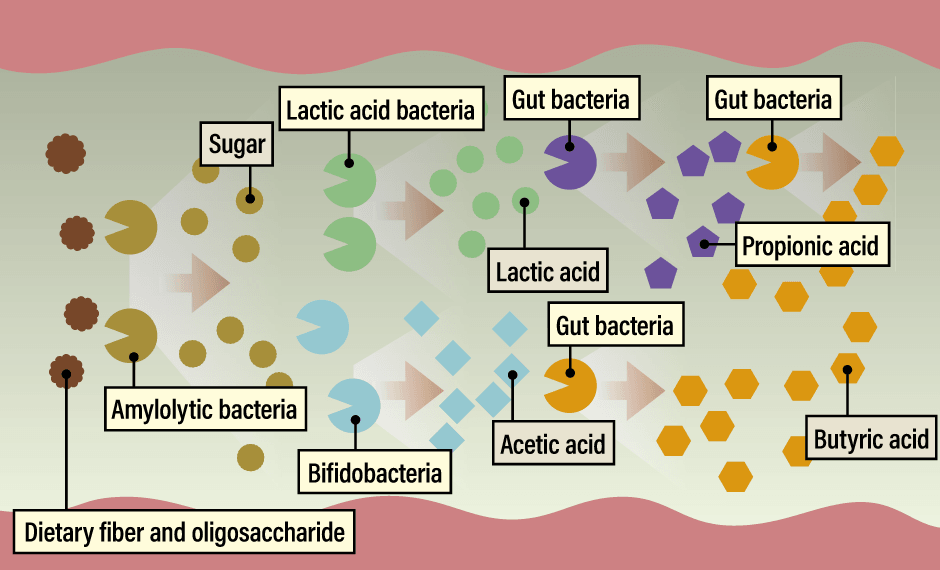
Figure 2. The bacterial relayThe gut microbiota is not improved simply by consuming large amounts of dietary fiber or oligosaccharides. Bacterial metabolism is important not only for the initial dietary substances, but also for the postbiotics that are metabolized and produced by the relay of multiple bacteria.
For example, people with few amylolytic bacteria in their intestines may not be able to produce sugar no matter how much fiber they consume, which is supposed to feed the intestinal bacteria, and instead may have a poor digestive system. In addition, people whose intestinal microbiota is low in bifidobacteria, which is one of representative of acetic acid-producing bacteria, have difficulty using the sugar produced by the amylolytic bacteria to make acetic acid, at which point the bacterial relay stops. When this happens, the production of butyric acid, from which acetic acid is made, may not be possible.
Furthermore, not all bacteria can metabolize all dietary fibers and oligosaccharides to the same extent. Even Blautia cannot metabolize certain one. Some studies have shown that bifidobacteria break down sugars and convert them into substances that are easier for Blautia to metabolize. Bifidobacteria would then play a role in supporting Blautia, and efforts to increase Blautia would be more effective if dietary fiber and oligosaccharides were consumed with Bifidobacterium.
Postbiotics produced by relay between bacteria
In the process of exploring this bacterial relay and the numerous postbiotics generated in order to shed light on the mechanisms, we reported in 2022 that alpha KetoA (αKetoA) might possibly inhibit the symptoms of allergic dermatitis (Figure 3).
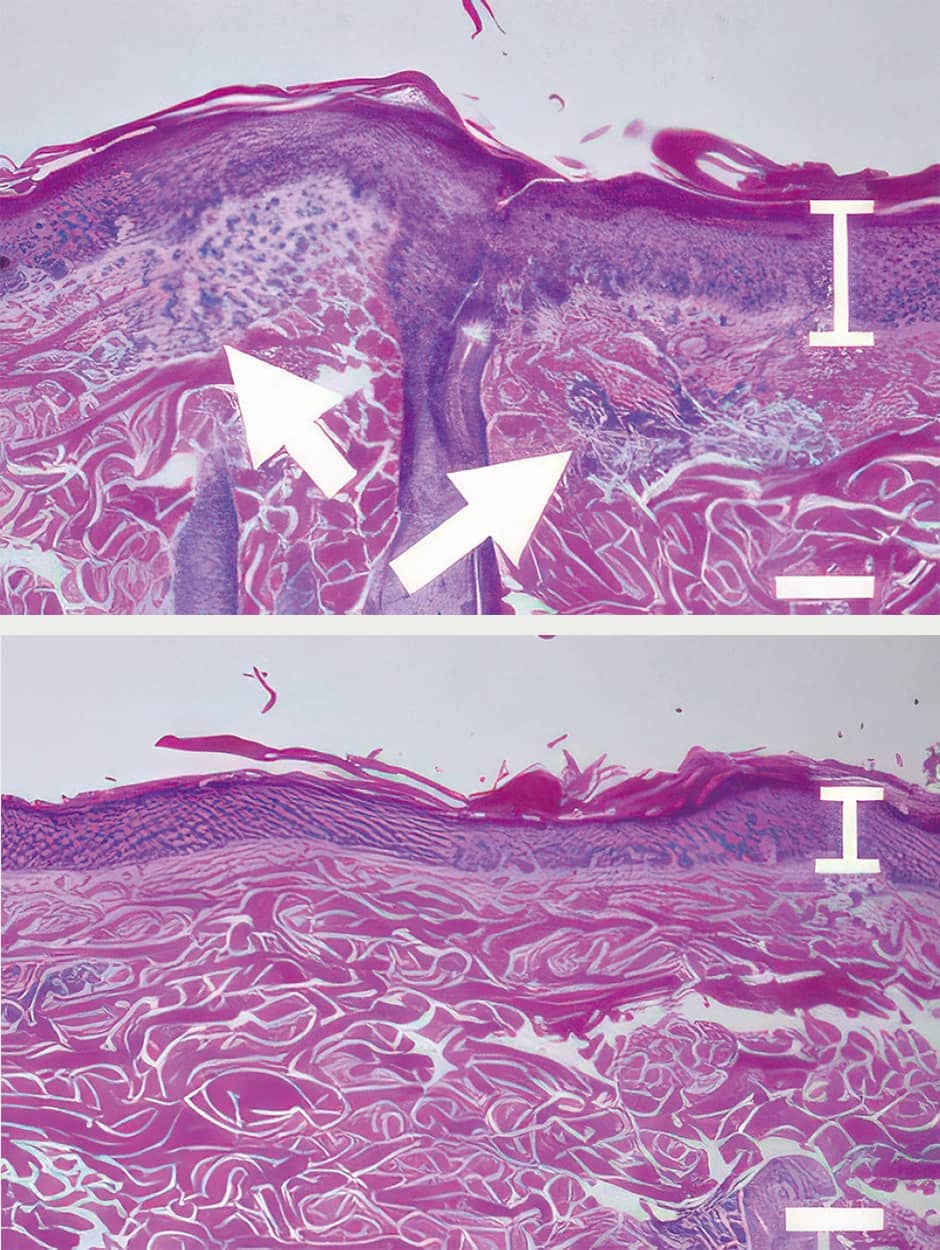
Figure 3. The effect of αKetoA in ameliorating dermatitisThe image on the left shows skin inflammation with excessive accumulation of immune cells. The image on the right shows that the administration of αKetoA has ameliorated the inflammation.
The ingredient from which αKetoA is derived is alpha-linolenic acid (ALA), an omega-3 fatty acid found in linseed oil and perilla oil, among others. Certain intestinal bacteria, including lactic acid bacteria, produce alpha-hyaluronic acid (αHYA) by metabolizing ALA, and then other bacteria convert this into αKetoA. A bacterial relay takes place here, too.
In human, while fecal αKetoA levels basically increase with intake of ALA, there are differences in level of around a hundredfold between individuals. If the level of αKetoA is low even if the same quantity of ALA is ingested, we can presume that the individual has a low level either of the bacteria that produce αHYA from ALA or of the bacteria that generate αKetoA from αHYA.
Gut bacteria have a symbiotic relationship with other bacteria similar to their symbiosis with humans. Accordingly, even though we have identified beneficial postbiotics, it does not mean that there is nothing to worry about as long as a person has the bacteria that produce these postbiotics.
Furthermore, what we can do to support the symbiotic relationships between gut bacteria and reap their benefits is to consume “good” bacteria. This is where fermented foods come in. Foods rich in lactic acid bacteria and other such beneficial bacteria, such as natto and yogurt, are useful for many people.
Dietary fiber, which serves as food for key bacteria, is also essential. Our analysis shows that Blautia needs to make up at least 6% of the microbiota to show a preventive effect on obesity and diabetes. On the other hand, our survey showed that Blautia is present in many Japanese people, but in most cases the level is below 6%, indicating a lack of dietary fiber. We therefore had the subjects work on improving the balance in their diet, including addressing the fiber deficiency, with the result that their Blautia levels reached an average of about 7.5% a year later. When we followed up later, some people had maintained high levels, while others had returned to low levels. In other words, since the levels of bacteria in our guts rise or fall depending on what we eat, it is important to continue to pay attention to what we eat.
A large body of data also shows that the composition of the gut microbiota changes with diet. Figure 4 shows the composition of the gut microbiota; an unbalanced diet skewed toward a few types of foods would be expected to increase only the bacteria that feed on those foods. Conversely, consuming a diet that includes a variety of food types will result in an increase in the gut bacteria that feed on these different foods, thereby increasing bacterial diversity.
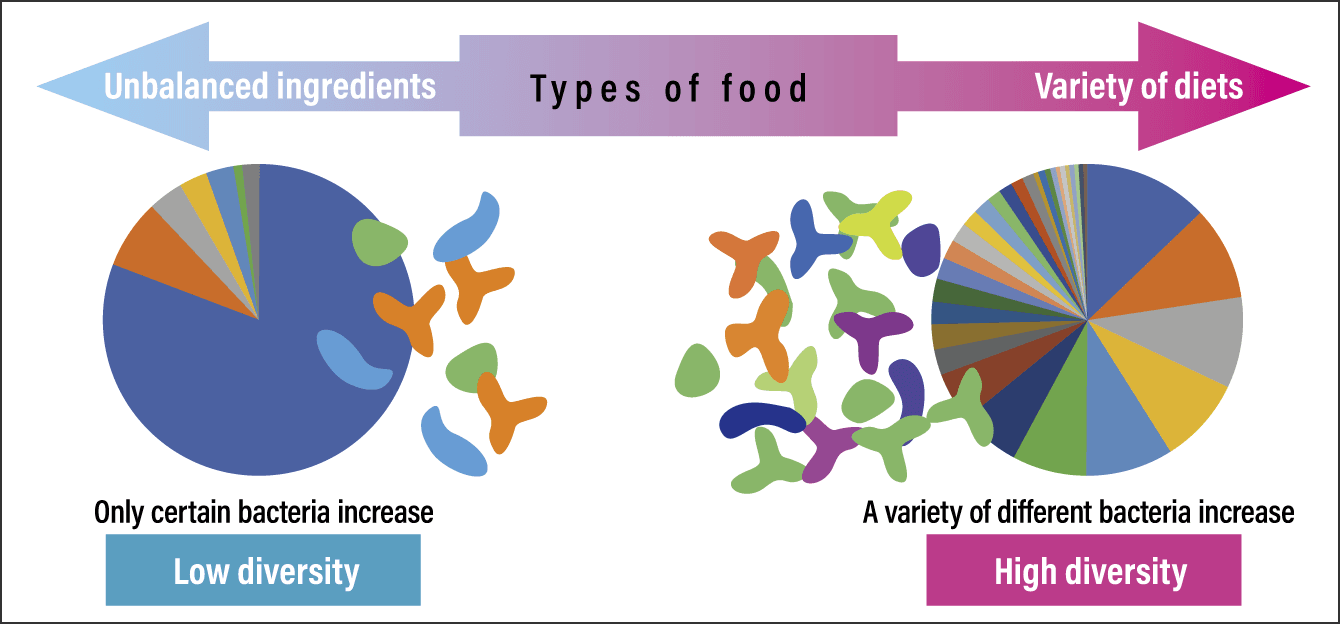
Figure 4. Dietary influences on intestinal bacterial diversityThe image on the right represents a balanced and diverse gut microbiota. In the figure on the left, the bacteria are less diverse, with one particular gut bacterium making up the majority of the microbiota. This may be due in part to the influence of the diet on which the gut bacteria feed.
Develop a simple, inexpensive and fast system to test for the presence of specific bacteria
The most important thing to focus on at this time is the diversity of the gut bacteria. A state of low bacterial diversity and extreme bias toward certain bacteria is called “dysbiosis” and is thought to be the cause of various diseases. In a situation where only certain types of gut bacteria are present, the bacteria that are present may function well at what they are good at, but the microbiota as a whole becomes deficient in functions that cannot be performed by these bacteria. In a diverse gut microbiota, each species of bacteria can perform its function and work with other bacteria to enable the microbiota as a whole to respond to a variety of situations. Also, the presence of diverse bacteria will increase the variety of postbiotics, and a bacterial relay will be established in any situation.
In other words, what we should ingest may depend on what and how many bacteria are present in our own gut. For example, fermented foods are the easiest way to cultivate beneficial bacteria, but if bifidobacteria make up a large part of our gut microbiota, we probably don’t need to eat any more yogurt than we already do.
So how many bacteria do you have in your own gut? Recently, a number of services have emerged to test an individual’s gut bacteria. However, these are expensive and time-consuming, making them not very user-friendly for those who want to easily find out what gut bacteria they need right now.
To resolve this, we are developing a system that produces antibodies that react to different types of gut bacteria and uses it to check for the target bacteria. This allows effective visualization of intestinal bacteria. It is already possible to test important intestinal bacteria in just one day, including Bifidobacterium, Blautia, Prevotella, Faecalibacterium, and Bacteroides. Research is currently underway with the goal of commercializing this technology by the end of 2024.
Understanding the composition of gut bacteria will also allow us to notice the necessary gut bacteria. If they are found to be deficient, people will be more careful in their food choices and can be tested again to see the effects of their diet. Since gut bacteria are also closely related to immunity, such a test would be useful for health management.
In addition to shedding light on the mechanisms of symbiosis among gut bacteria and the effects of various postbiotics, individual differences in gut microbiota have also been observed. Scientists used to think that body shape and constitution were determined by genes, but now recognize that many healthy conditions are determined by gut bacteria. So we need to understand that gut bacteria are as important as genes in determining our health, and we need to be aware of their importance in our diet.