Less than 10% of polyphenols are absorbed in the small intestine, with the vast majority moving into the colon. The potential for polyphenols to have a direct effect on metabolism is therefore low; scientists think that gut bacterial activity changes the structure of polyphenols, turning them into potent active substances with new functions. At the same time, scientists have discovered that polyphenols also have an effect on gut bacteria. Phenols produced by gut bacteria are the root cause of diseases including uremia, diabetes, and diabetic kidney disease, but scientists say polyphenols can regulate them. Attention is now focusing on the interaction between polyphenols and gut microbes.
Special Feature 1 – The Mysteries of Polyphenols Interaction with gut bacteria turns polyphenols into potent active substances
text by Yumi Ohuchi
In recent years, attention has begun to focus on the relationship between polyphenols and the gut microbiota. This is because it has become apparent that the absorption rate of polyphenols in the small intestine is less than 10%, and more than 90% moves on to the colon, where some are metabolized by gut bacteria and then circulate around the body. In other words, scientists say there is a possibility that the functions of polyphenols believed to be good for our health are expressed at a higher level through interaction with gut bacteria than through metabolic pathways around the body resulting from absorption in the small intestine.
Associate Professor Kyuichi Kawabata of the Faculty of Clinical Nutrition and Dietetics at Konan Women’ s University is conducting research focused on the functional interaction between the gut microbiota and polyphenols.
Gut bacteria decouple structures to create low molecular weight compounds
“Gut bacteria change the structures of polyphenols,” Kawabata says. “These structural changes also affect the polyphenols’ functions.”
One such change known to occur due to the modification of their molecular structure results in some polyphenols being transformed into substances that demonstrate new or more powerful activity than the original polyphenol (parent compound). For example, isoflavones, a class of polyphenols found in soybeans, are known to mimic the effects of a female hormone. Equol, which is produced when the isoflavone daidzein undergoes metabolic conversion by gut bacteria, has a higher ability to bind to estrogen receptors than daidzein, and is thought to play a large part in these estrogen-like effects.
However, cases in which they become converted metabolites of this kind are rare; most polyphenols have their structures decoupled by gut bacteria, and are then metabolized into compounds with a low molecular weight.
“When polyphenols are metabolized into low molecular weight compounds, they potentially lose their expected or confirmed functionality as polyphenols,” Kawabata explains. “On the other hand, there have also been reports of low molecular weight compounds being present in blood in higher concentrations than the parent compounds, so we can’t deny the possibility that these low molecular weight compounds are responsible for polyphenol functionality.”
Health functions increase via combination with beneficial bacteria
Kawabata points out the possibility that, in addition to demonstrating functions in their own right, polyphenols that have been structurally modified by gut bacteria might also have an impact on the gut microbiota.
“Based on existing research reports, it’s conceivable that consumption of polyphenols could be involved in changes in the gut microbiota, such as increasing beneficial bacteria and reducing harmful bacteria, as well as being involved in the production of gut bacteria metabolites, including short-chain fatty acids and tryptophan. Another thing on which we focused was the regulation of gut bacteria functionality by polyphenols. We were hopeful about the possibility that health functions would increase as a result of combining polyphenols with beneficial bacteria, so we examined their combined effect as an indicator of anti-inflammatory activity.”
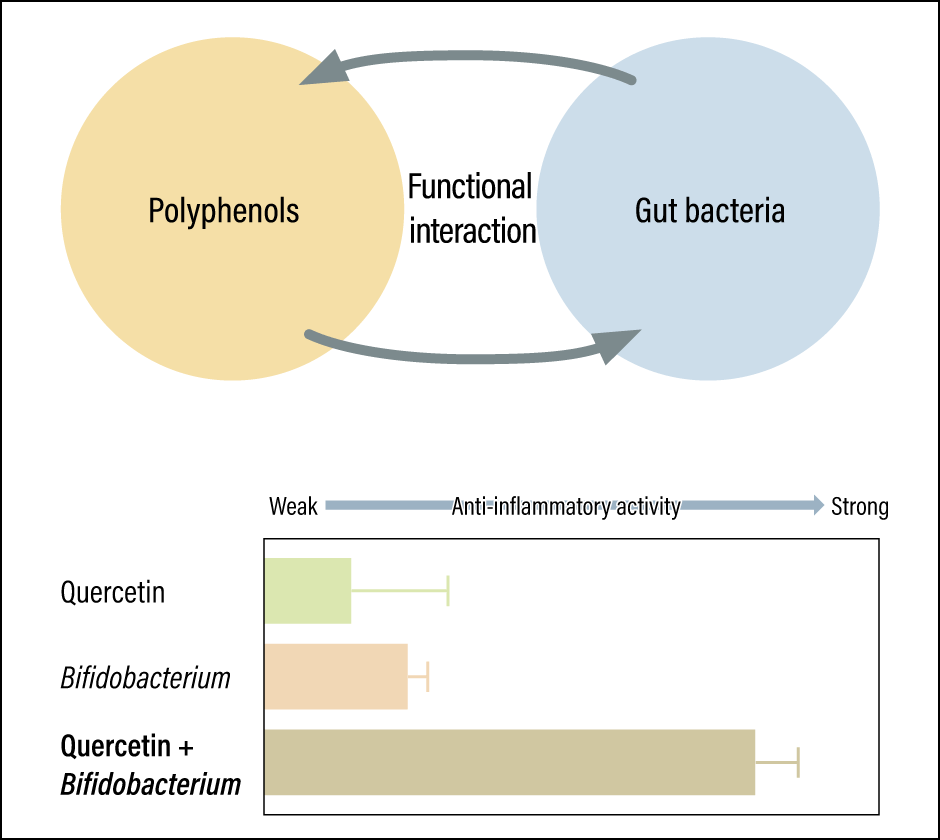
In an experiment conducted by Kawabata’s research team, quercetin and four polyphenols with a similar structure to quercetin, three Bifidobacterium species, and three Lactobacillus species were cultured either separately or in combination. The researchers then checked the anti-inflammatory activity of the culture supernatant (the clear layer of fluid at the top of the solution). The polyphenol-only and Bifidobacterium– and Lactobacillus-only cultures showed either weak or virtually no anti-inflammatory activity (Figure 1).
 Compiled with reference to Kawabata K. et al., 2013; 2015; 2018; 2019
Compiled with reference to Kawabata K. et al., 2013; 2015; 2018; 2019
Figure 1. Functional interaction of polyphenols and gut bacteriaWhile quercetin and Bifidobacterium each demonstrated weak anti-inflammatory activity on their own, powerful activity was observed when quercetin and Bifidobacterium were combined.
“As we’d hoped, we confirmed that combining quercetin and Bifidobacterium —— specifically, Bifidobacterium adolescentis (B. adolescentis) —— markedly increases anti-inflammatory activity,” Kawabata says. “The fact that we got this result by combining B. adolescentis, which is resident in most healthy adults, with quercetin, which most fruit and vegetables contain, meant it was an extremely interesting discovery.”
Further research focused on this combination also indicated that the potency of activity increases with longer culture duration and higher quercetin concentrations.
“We thought that quercetin might be metabolized by B. adolescentis into a more active substance, as in the case of equol,” Kawabata continues. “However, looking at anti-inflammatory activity after increasing the number of B. adolescentis, we found that even increases in the number of uncombined bacteria resulted in more potent activity, although less than when quercetin was added, and that B. adolescentis itself has potential anti-inflammatory effects. In short, we surmised that the component involved in anti-inflammatory activity enhanced by the combination of the two is not a metabolite derived from quercetin.”
Anti-inflammatory activity hardly changed at all when the research team removed the polyphenols from the quercetin and B. adolescentis culture mediums that showed anti-inflammatory activity.
“From these results, it appeared that quercetin enhances the potential anti-inflammatory activity of Bifidobacterium,” Kawabata explains. “This indicated the possibility of a new functional interaction, whereby polyphenols imbue gut bacteria with high functionality.” (Figure 2)
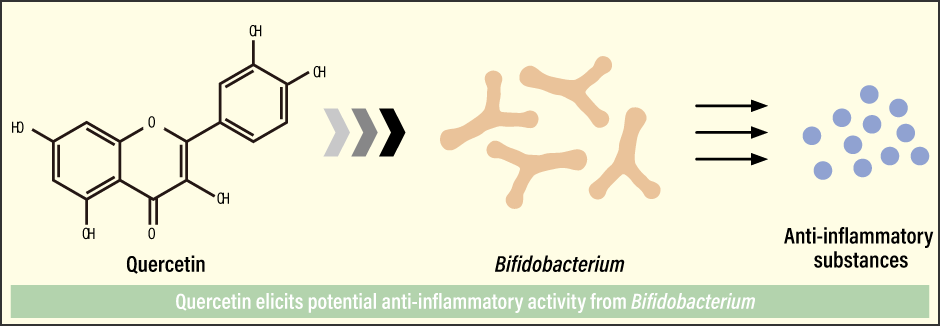
Figure 2. Activation of Bifidobacterium by polyphenolsThis reportedly indicates a new functional interaction, whereby polyphenols imbue gut bacteria with high functionality.
Kawabata is currently undertaking analysis aimed at shedding light on the molecular mechanism involved. So far, in addition to identifying candidate anti-inflammatory substances secreted by Bifidobacterium, he has ascertained that quercetin might possibly alter Bifidobacterium gene expression. However, as diet and the gut microbiota vary from one individual to another, he says that he is also in the process of planning experiments using human gut microbiota.
“Given the existence of people in whom equol production, for example, is also difficult, it’s not necessarily the case that this functionality will be demonstrated. I believe it will be crucial to tailor consumption in a way that takes into account each individual’s diet and gut microbiota —— for example consuming fruit along with dairy products that contain bifidobacteria.”
“We might also observe the increased functionality of gut bacteria conferred by polyphenols in health functions other than anti-inflammatory activity,” he adds.
There are many different types of both polyphenols and gut bacteria, which will make for an extraordinary number of combinations. Kawabata plans to continue his research with a focus on quercetin and other polyphenols that can readily be consumed in our everyday diets, and the gut bacteria prevalent among Japanese people.
Polyphenols inhibit phenol production by gut bacteria
There are also research findings concerning the effects of polyphenols on gut bacteria that could lead to the prevention of disease. Disruption of the gut environment can cause disease; in particular, the phenols (present in the blood in the form of the sulfate conjugate phenyl sulfate) produced by gut bacteria are known to be uremic toxins. It has been reported that phenols are the root cause of not only uremia, but also diabetes and diabetic kidney disease, and that they are involved in skin aging (skin roughness and dryness), and gastrointestinal neurological symptoms such as constipation and diarrhea, as well.
Phenols are produced when tyrosine —— a type of amino acid derived from protein in food —— is metabolized by the enzyme tyrosine phenol-lyase (TPL), which is expressed in some gut bacteria. Studies have indicated the possibility that inhibiting TPL could be effective in treating phenol-induced symptoms, including uremia, and 2-aza-tyrosine (2-aza-Tyr) has already been developed as a TPL inhibitor.
More recently, a research team led by Associate Professor Noriyuki Miyoshi at the University of Shizuoka’s School of Food and Nutritional Sciences has discovered that polyphenols have a TPL inhibitory effect.
“We’ve been conducting research into phenols as biomarkers and functional analysis of foods, aimed at achieving the prevention and early discovery of disease,” Miyoshi explains. “We wondered whether it would be possible to regulate phenols using polyphenols consumed in food.”
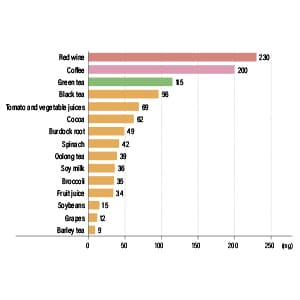
As part of this research, the team created a gene recombinant from gut bacteria that express TPL, arranged a situation in which phenols would be produced, and explored the inhibitory effects of polyphenols against them. The polyphenols on which the study focused are all components of our everyday diets, including quercetin —— the most common —— and polyphenols found in green tea, coffee, and curry spices (Figure 3).
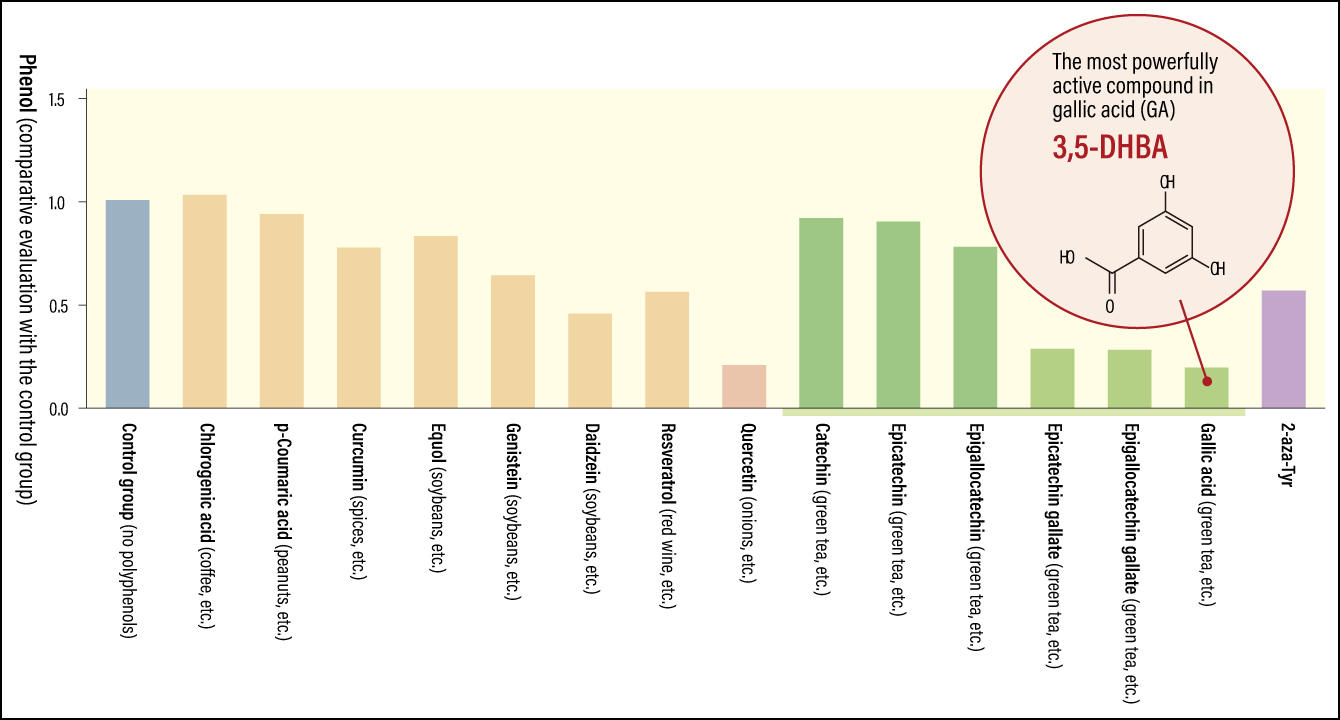 Modified from Kobayashi T, et al. PNAS Nexus, 3: page 265, 2024
Modified from Kobayashi T, et al. PNAS Nexus, 3: page 265, 2024
Figure 3. TPL inhibitory activity of polyphenolsThe researchers found that gallic acid (GA) demonstrated the most potent TPL inhibitory activity, with its 3,5-dihydroxybenzoic acid (3,5-DHBA) structure demonstrating particularly potent inhibitory activity.
“The strongest TPL inhibitory effect and greatest reduction in phenols was observed in the cases of quercetin and three polyphenols found in green tea, namely epicatechin gallate (ECG), epigallocatechin gallate (EGCG), and gallic acid (GA),” Miyoshi says. “What’s more, the activity of these polyphenols was even more potent than the established TPL inhibitor 2-aza-Tyr. We then proceeded to undertake further analysis of GA, which demonstrated the most potent inhibitory activity of all.”
GA, too, is turned into low molecular weight compounds with a large, diverse array of structures as a result of gut bacterial metabolism. When Miyoshi’s team analyzed which of the isomers expressed the most potent TPL inhibitory activity, a particular structural pattern indicative of inhibitory activity became apparent, he says.
“We found that, of all the isomers, 3,5-dihydroxybenzoic acid (3,5-DHBA) demonstrated the most potent TPL inhibitory activity. X-ray crystallography also enabled us to use 3D images to confirm that 3,5-DHBA inhibits TPL.”
Not being absorbed actually makes them more effective
Additionally, the researchers created culture media providing conditions in which a large quantity of phenols would be produced, by adding tyrosine to the TPL-expressing gut bacteria Morganella morganii and Citrobacter koseri. When 3,5-DHBA was placed in these culture media, the quantity of phenols produced in both media declined in a 3,5-DHBA-concentration-dependent manner. Then, when the researchers administered 3,5-DHBA to mice that had been fed a tyrosine-rich diet to increase the quantity of phenols they produced, the level of phenols in the feces of the mice fell, Miyoshi says.
“From these results, we believe that 3,5-DHBA could become a new TPL inhibitor. The polyphenol GA —— the parent compound of 3,5-DHBA —— is part of our everyday diet, as it’s found in green tea, as well as fruit and vegetables. We can also expect quercetin to serve as a TPL inhibitor, as demonstrated by the results of our experiments.”
Miyoshi’s research team is also exploring ways to measure phenol concentrations in breath and via the surface of the skin, rather than in blood and feces.
“If we can find a simple way of measuring phenol concentrations, we’ll be able to assess people’s risk of diseases such as diabetes, as well as symptoms caused by uremic toxins, including nausea, dizziness, headache, decreased appetite, and skin problems,” he continues. “If someone’s phenol concentration is high, we’ll also conceivably be able to adopt the approach of consuming polyphenols as a TPL inhibitor, in order to improve or prevent symptoms.” (Figure 4)
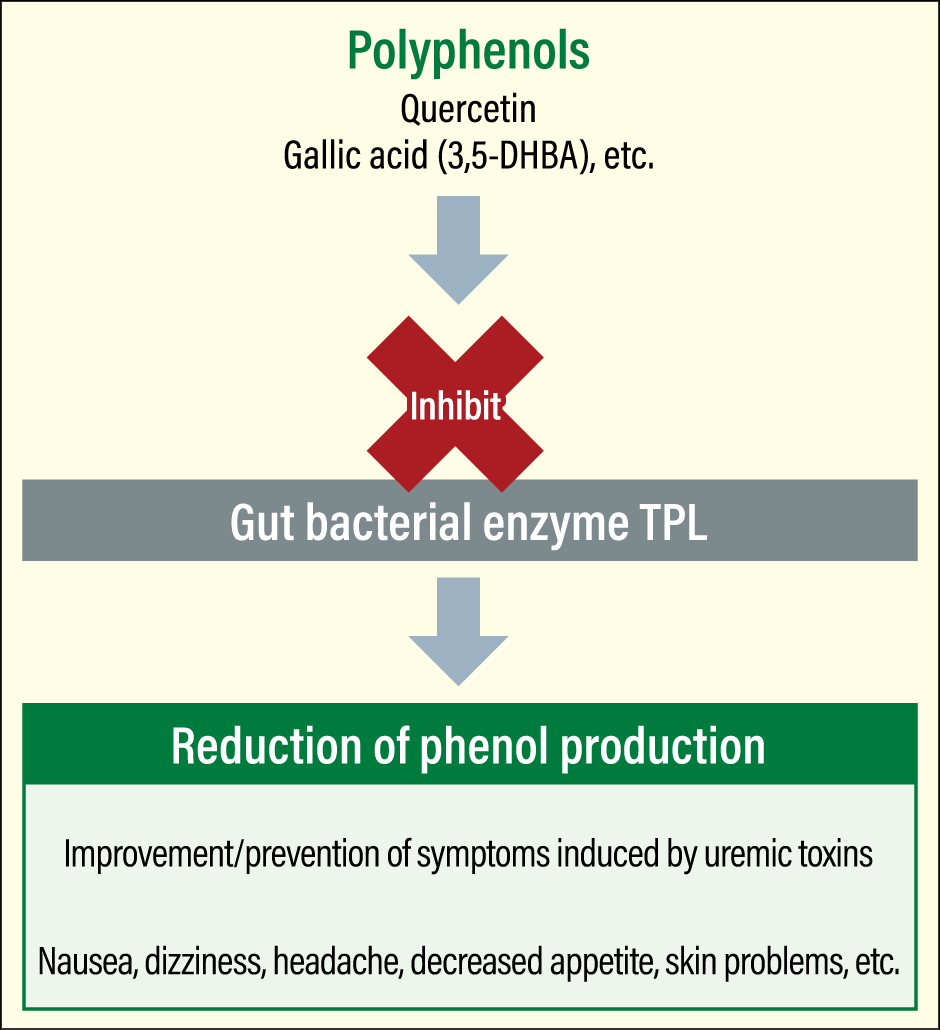
Figure 4. Use of a polyphenol-derived TPL inhibitorIf phenol concentrations are high, consuming polyphenols as a TPL inhibitor could possibly lead to the improvement or prevention of symptoms.
Miyoshi has something else to say about the significance of research into polyphenols and gut bacteria.
“Nonnutritive food components such as polyphenols often show low rates of absorption in the body. However, if these food components have an effect on gut bacteria, there’s no need for them to be absorbed, and not being absorbed actually makes them more effective. It’s probably fair to say that these properties will be subject to further food function research.”
Both the scientists we spoke to were kind enough to show us the findings of studies from Japan and overseas alike, including their other research. It is anticipated that hitherto-unknown functions of polyphenols and gut bacteria resulting from their interaction will continue to emerge in due course. When seeking to tap into these functions, we may well need to do our own bit by maintaining a good diet and gut environment.