Since the beginning of the 2000s, the emergence of next-generation sequencers has made large-scale genome analysis possible, resulting in dramatic advances in research into enteric bacteria and other microorganisms indigenous to the human body. However, hardly any of the approximately 9,000 types of viruses said to infect humans are understood as yet. So far, it has become clear that there are 39 types of viruses in the human body, but many aspects of how they affect our bodies and health remain unknown. Hopes for future research are high.
Special Feature 1 – The Unknown World of Viruses The relationship between humans and viruses —— promising field for future research
composition by Yumi Ohuchi
Recent years have seen advances in metagenomic analysis, the process of comprehensively analyzing all nucleic acid (DNA and RNA) in the tissue of animals and plants. Virology researchers, too, are attempting to analyze the viral component of the microbiome (i.e., the “virome”) to exhaustively identify the viruses present in such samples. The objective in most cases is to find unknown viruses that cause infection as human or animal pathogens. For instance, when a patient has a fever of unknown cause and an infectious disease is suspected, their tissues are searched for the presence of viruses. Scientists also search for viruses prevalent among wild animals that could in the future cause infectious diseases in humans. A worldwide initiative undertaking such efforts, the Global Virome Project estimates that, whereas only around 9,000 viruses causing infection in humans have been defined to date, there are between approximately 630,000 and 830,000 virus species capable of doing so, which are yet to be discovered.
Virome research focused on diseases including the novel coronavirus COVID-19 is progressing, and scientists in Japan have discovered new viruses such as Yezo virus. As infectious diseases are a threat to humanity, there is an inevitable tendency to focus on viruses as pathogens, but in fact, it has long been known that viruses are also present in healthy humans.
- * Yezo virus : A tick-borne virus discovered by a team of researchers from institutions including the Hokkaido University International Institute for Zoonosis Control. The discovery was published in 2021. The virus is believed to cause an infectious disease whose primary symptoms include fever and muscular pain.
39 virus species are found in healthy humans
For example, herpes viruses are widespread in the natural world, infecting vertebrates such as fish, amphibians, and mammals. The Epstein-Barr virus (EBV), a member of the group of human herpesviruses, is believed to infect almost 90% of adults, but only rarely causes symptoms, which include fatigue and fever. This mode of infection, where the virus infects the host and proliferates inside it without causing symptoms, is called an inapparent infection.
The mode of infection whereby a virus —— regardless of whether it causes symptoms once it has infiltrated a body —— temporarily stops the proliferation cycle and remains in organs or tissues, but then reactivates when immune function is reduced for some reason, is called a latent infection. This is a feature of almost all the aforementioned herpes viruses.
Our bodies are thought to experience inapparent or latent infections by a variety of other viruses as well, and these could potentially have some kind of impact on human tissues. However, the extent to which viruses are present in healthy humans and the organs where they are found was not known, as it is difficult to collect organ tissue from humans who are not sick. Accordingly, Ryuichi Kumata —— then a graduate student in my research team —— led the world’s first investigation of the virome in a full range of healthy human tissues. We used as samples the RNA-sequencing dataset from the Genotype-Tissue Expression (GTEx) project, a huge genome project in the U.S. This is because, when someone is infected with some kind of virus, the virus’s RNA proliferates, so it is envisaged that viral sequences will also be included in the RNA-sequencing data.
The data was obtained only from those dissections of the cadavers in the U.S., where infectious disease had not been the cause of death. The original purpose of the GTEx project was to shed light on how genetic polymorphisms (differences in nucleotide sequences among individuals) affect tissue-specific gene expression. The project has made available a public database containing images of microscopic specimens of organ tissue and RNA-sequencing data collected from 54 non-diseased tissue sites across nearly 1,000 individuals. With more than 10,000 samples, amounting to 100 terabytes of data, this is an example of big data. Accordingly, we used our institute’s SHIROKANE supercomputer to analyze the data. Enabling data amassed through past genomic research to be reused, it might even be fair to say that this study could only have been carried out now that data analysis technology has advanced so far.
We first used genomic information for 5,139 virus species registered with the U.S. National Center for Biotechnology Information (NCBI) to search the GTEx data for RNA viral sequences. As a result, we detected 39 viral species and clarified the positivity rate (frequency of detection) of each virus in each organ (Figure 1).
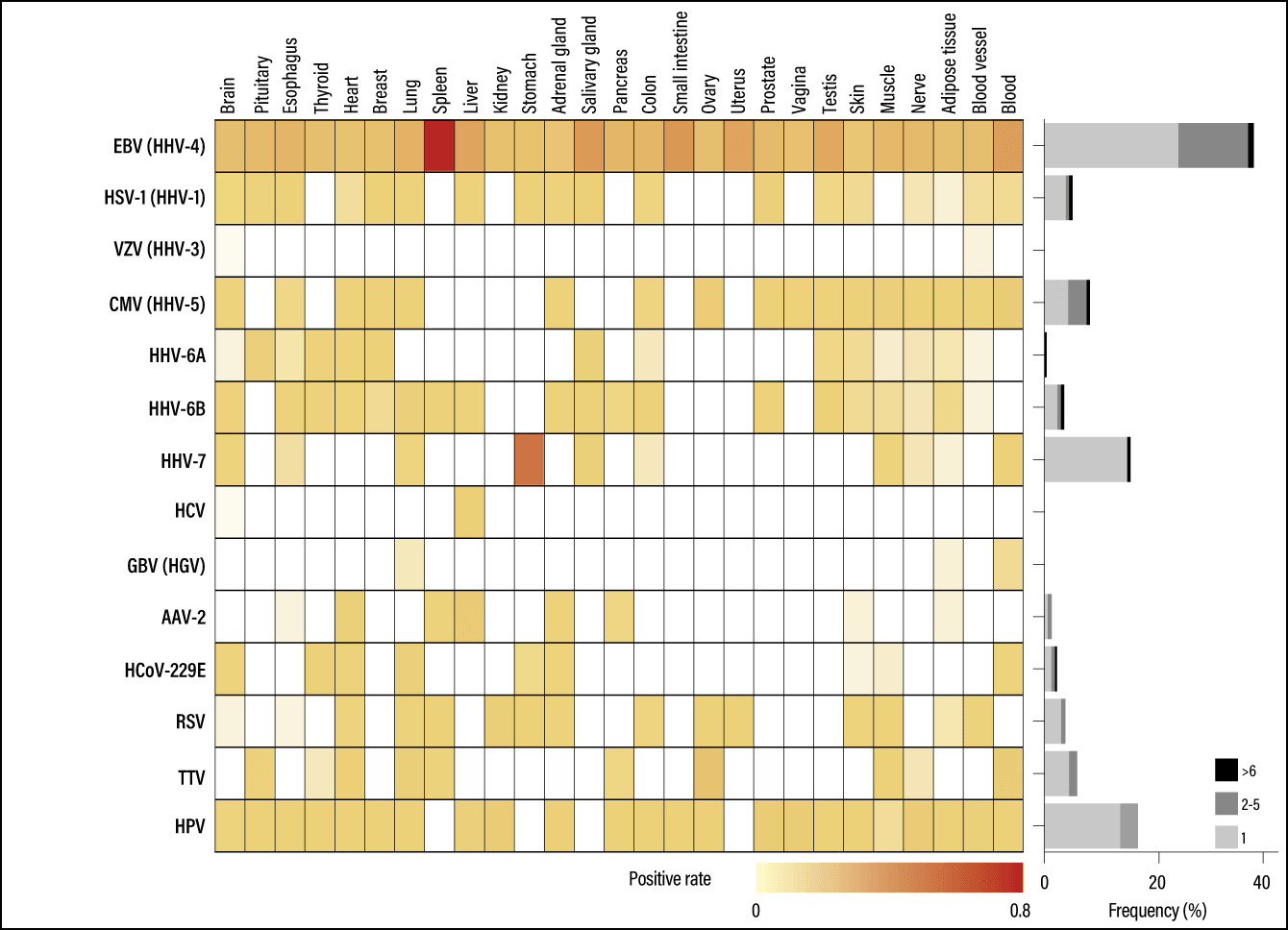 (Modified from Kumata R, et al. BMC Biology (2020) 18:55)
(Modified from Kumata R, et al. BMC Biology (2020) 18:55)
Figure 1. The main viruses detected and their locationsPositive rate of each virus in each human tissue. Darker colors indicate higher positive rates. The bar chart indicates the frequency of specimens that were positive for each virus.
Most of the viruses detected are commonly known to infect humans. They include the aforementioned human herpesviruses; a type of human coronavirus that causes seasonal colds; the respiratory syncytial virus (RSV), which infects infants and young children, causing bronchitis and pneumonia, among others; the human papillomavirus, which is involved in the onset of cervical cancer; and the hepatitis C virus (HCV). There was nothing surprising about these results, which could be said to reflect existing knowledge. However, we also detected viruses that are frequently found in healthy humans but have not been shown to be pathogenic.
At the same time, we also detected viruses that infect non-human vertebrates, insects, and plants, among others. However, the positivity rates for viruses that infect organisms other than humans are low, suggesting a strong possibility that they just happened to be in the tissue, rather than having infected the individual.
For example, we found a virus that infects tomatoes (tomato spotted wilt virus), which might have remained in the stomach or other tissue after the individual ate an infected tomato.
Looking at whether or not a virus was present in specific tissues, we discovered that some viruses, such as EBV and herpes simplex virus type 1 (HSV-1), were found throughout the body or in the majority of tissues, whereas others were present in a tissue-specific manner.
In fact, before commencing this study, we hypothesized that a larger number of viruses would be detected at a higher frequency. For example, we expected to see viruses that infect non-human animals, with the potential to cause zoonoses. However, we did not detect any viruses with such powerful impacts, which one could say is a good result for humanity.
Effects on human immune response and physiological function
In this study, we also analyzed the relationship between human gene expression and the presence or absence of viral genes, in order to investigate the effects of viruses present in the human body. As a result, we found certain characteristics in human immune responses associated with a number of viruses (Figure 2).
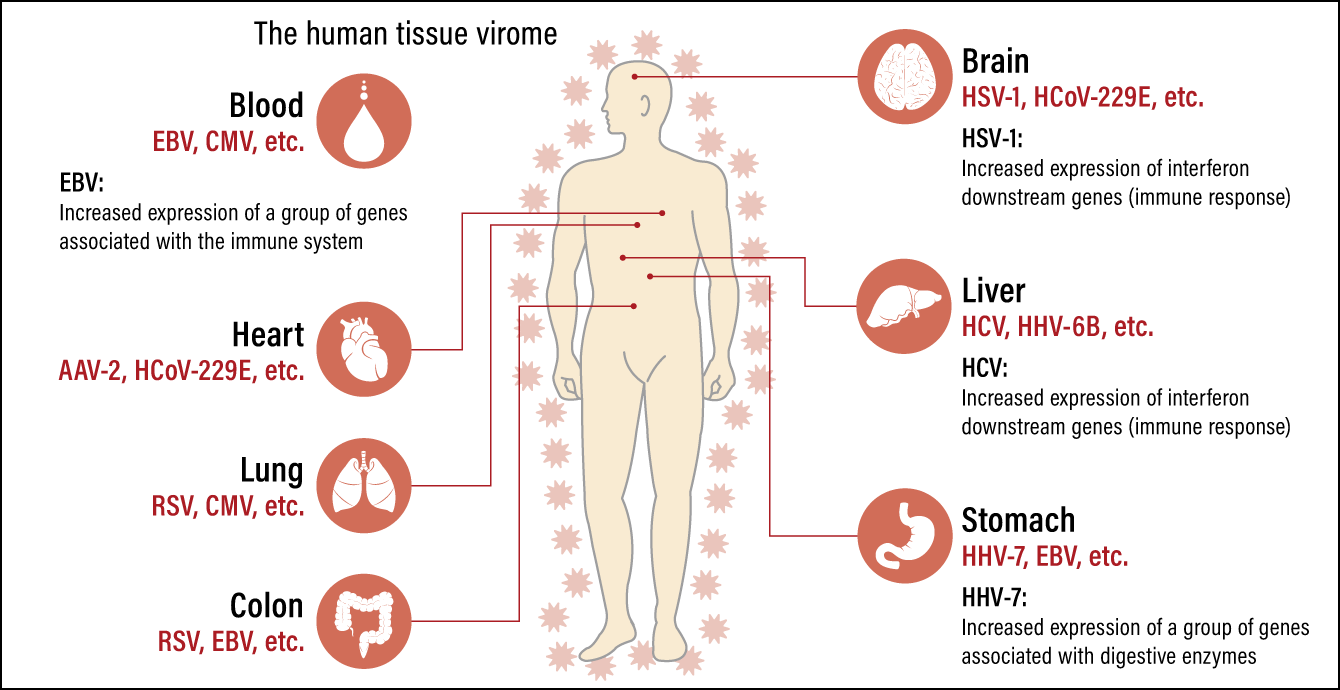
Figure 2. The main viruses detected and their relationships to humansVarious types of viruses are present in healthy individuals, including in the brain, lungs, heart, digestive organs, blood, and nerves. Among them are viruses indicating possible relations to organ specificity, strong immune responses or organ conditions.
One such virus is HCV, which demonstrated organ specificity, as it was hardly detected in any organs other than the liver. Of the 136 liver samples, three were HCV-positive and demonstrated a pronounced increase in the expression of a group of genes (downstream genes) regulated by interferon, which is a protein secreted by immune cells. Interferon is produced in the body by lymphocytes and other cells in the event of a viral infection and the expression of these downstream genes is regarded as a marker for measuring the strength of the innate immune response. In other words, the HCV-positive samples were demonstrating a strong immune response.
Moreover, when we had a pathologist examine the images of the microscopic specimens of the HCV-positive samples, it was found that the tissue was presenting symptoms of hepatitis. This suggests the possibility that, although it was not the direct cause of death, the patient was infected with HCV and had developed hepatitis.
HSV-1 is another virus to which a similarly strong immune response was demonstrated. HSV-1 is known as the virus that causes oral herpes, which results in the formation of small blisters on and around the lips. However, if HSV-1 reaches the brain, it can cause encephalitis, which can be fatal. High levels of HSV-1 RNA were detected in brain tissue in one sample, with a pronounced increase in the expression of interferon downstream genes. The subject is presumed to have developed encephalitis. Additionally, in EBV-positive samples, there was increased expression of a group of genes associated with the immune system in the blood and spleen, which contain a large number of the B cells believed to be the main cells infected by EBV.
Viruses of the kind described above are known to demonstrate an obvious pathogenesis. But what about viruses that are not known to be markedly pathogenic? For example, the pathogenesis of torque teno virus (TTV) is unclear. It is known to be detected in healthy humans and was detected in multiple organ samples in our study. Comparing samples that are positive and negative for TTV, we discovered that there was no difference between them in the expression of interferon downstream genes. In other words, the virus is thought to cause inapparent infection in the body, while evading the human immune response. We cannot tell from this result, but there is a possibility that TTV might have some kind of effect in humans.
Furthermore, we observed a virus that might possibly affect not only immune response, but also physiological functions in humans. This is human herpesvirus 7 (HHV-7), which is known to cause roseola infantum (also known as exanthema subitum) in infants. We know that most adults are latently infected, as they have HHV-7 antibodies, but the virus’s pathogenesis in adults is not clear.
In our study, too, HHV-7 was detected in tissue from several organs, including the blood, brain, lungs, and muscle, with a notably high positivity rate in the stomach. This was the first time HHV-7 had been detected in the stomach.
In addition, we discovered major differences in gene expression patterns in the stomach between HHV-7-positive and negative stomach samples. Furthermore, when we investigated which genes demonstrated differences in expression, we found that the expression of genes associated with digestive enzymes was considerably higher in the group with a high level of positivity. The following observations can be made regarding these results.
First, it would appear that either HHV-7 infection altered the gene pattern in the stomach, or, conversely, HHV-7 more readily infects stomachs that demonstrate a specific gene expression pattern. In addition, as it is not clear from this data which part of the stomach the samples were taken from, there is also a possibility that HHV-7 might infect only a specific part of the stomach. As this is an association analysis, we cannot tell whether there is a causal relationship, but there is certainly a possibility that HHV-7 infection is related to the condition of the stomach.
Virome research progressing due to COVID-19
Thus, our study partially uncovered the symbiotic relationship between humans and viruses. We believe that further advances will be possible if we apply the analytical techniques used in this study to a variety of RNA-sequencing data. For example, the data we used for this study was obtained from Americans of a variety of ethnicities, but it is conceivable that viromes differ by race and even sex. Depending on the data, it might be possible to conduct race- or sex-specific analyses.
In addition to identifying the pathogens responsible for infectious diseases of unknown origin, it is hoped that such analyses might shed light on the relationship between individuals’ propensity to develop diseases and their normal viruses. Specifically, this might be achieved through a comparison of virome data with information about the risk of developing diseases such as diabetes, for example, which at first glance do not appear to be related to viruses, but which might be affected by genetic polymorphisms.
Virome research had been lagging behind microbiome research which has been flourishing in recent years. One reason for this is the fact that, whereas all bacteria have a number of genes in common, viruses do not have any common genes. However, with the aid of the techniques used in our study, it is possible to conduct analyses targeting numerous viruses.
Scientists in the field of microbiome research say there are several hundred trillion bacteria present on human skin and in the human body. In contrast, while some theories estimate the number of viruses on human skin and in the human body at 380 trillion, most of these are actually said to be viruses that infect bacteria (phages). This phageome is also likely to be a highly interesting topic for research.
Great advances in virology are currently being made, due to the COVID-19 pandemic. Bioinformatics researchers work on solving the problems of life by analyzing data, as in our recent study, and many are now entering the field of virology. I hope that virome research will advance further in due course, shedding light on the symbiotic relationship between humans and viruses.




















