Statistical data shows that the incidence of autoimmune diseases, such as rheumatism and collagen disease, is higher in women than in men. In addition, the prevalence and severity of bronchial asthma also tends to be higher in women. Why is this? Connection with sex hormones first comes to mind, but in fact, the difference is said to be present from the stage of contact with allergens. Cells that control immune responses have sexes, and this leads to sex differences in pathological conditions.
Special Feature 1 – Diversity in Sex Asthma more severe in women than in men? Immunity has sex
text by Rie Iizuka
It is well known that individuals are more susceptible to specific diseases and are more prone to increased severity depending on their sex. The prevalence of cancers unrelated to genitals is overwhelmingly higher in men, while the incidence of immune-related diseases, such as autoimmune diseases, is higher in women. Some viral infections like coronavirus infections, SARS (severe acute respiratory syndrome) and MERS (Middle East respiratory syndrome) are reportedly almost twice as severe in men as in women on average.
Why such differences? Professor Isao Ohno at the Faculty of Medicine, Tohoku Medical and Pharmaceutical University explains as follows:
“The difference in susceptibility to diseases and pathological conditions between men and women is considered to be associated with sex-based differences in their daily lives, including their social environment and health consciousness. According to the National Health and Nutrition Survey, more women than men tend to make an effort to regulate their intestinal environment by, for example, ingesting lactic acid bacteria. Women eat fruits and vegetables more actively than men, while there are more smokers among men than women. Meanwhile, it is also known that women are more vulnerable to stress. As stress is clearly associated with the incidence of certain types of diseases, when we think about sex differences in illness, we must take into consideration the differences in lifestyles.
Our study subject, bronchial asthma, is also an example with marked sex differences. In Japan, the prevalence of asthma in adults is estimated at 6–10%, and the percentage is said to be increasing.
Two factors are greatly contributing to bronchial asthma. One is the patient’s environment, such as air pollution, smoking habits, and susceptibility to respiratory infections due to suffering from another disease. The other is the patient’s innate attributes, such as heredity and sex. While the pathological conditions of bronchial asthma are decided by interactions between the environmental factors and the individual factors, prevalence and severity tend to be higher in women than in men.
The number of deaths from asthma in Japan (2016; the number of deaths from asthma by age group) is high in women, particularly in women aged 75 or over. U.S. statistics also show a similar tendency, and when we look at European and U.S. data on the recurrence rate of asthma, the conditions tend to become more severe in women.
There are also characteristics based on age. The prevalence is higher in men until adolescence, but it starts to get higher in women from around the time when the adolescence ends (Figure 1).”
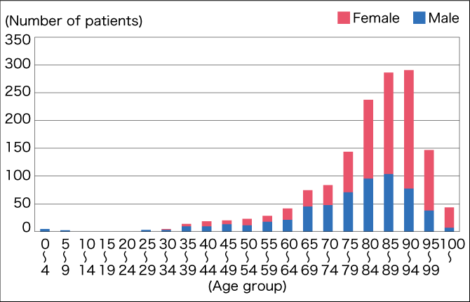
Figure 1. Period prevalence of respiratory allergy-like symptoms by sexIn other countries as well, male patients outnumber female patients in young age, but female patients exceed male patients after adolescence.
(Source: Period prevalence by age in “Survey on Trends in Health and Welfare” [2003])
Sex differences show from the moment of contact
Given that the prevalence in men and women reverses after adolescence, we could assume that the sex differences come from the effect of sex hormones. However, Dr. Tomomitsu Miyasaka, Lecturer at the Faculty of Pharmaceutical Sciences, Tohoku Medical and Pharmaceutical University, explains that in case of bronchial asthma, which is a runaway immune response, sex differences already start to show from the moment of catching allergens.
“Bronchial asthma is a disease that develops due to an immune response against allergens that enter the respiratory tract. The immune response consists of acquired immune response and innate immune response. Acquired immune response, characterized by formation of antibodies, is extremely powerful, but as the antibody formation takes time, innate immune response serves as a temporary stronghold in the meantime (Figure 2).
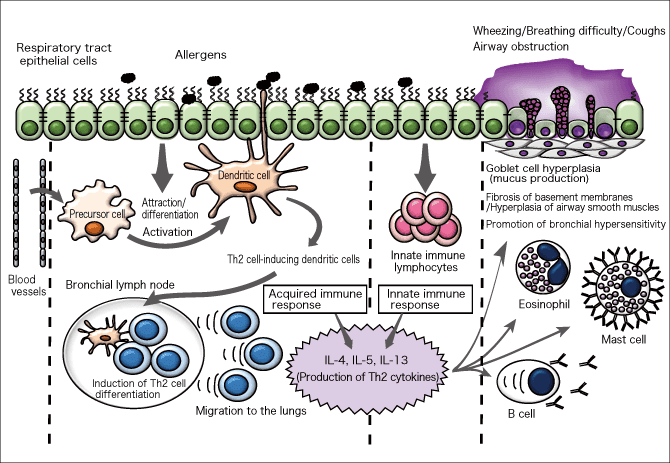
Figure 2. Pathogenic sequence of bronchial asthmaThere are no sex differences in the mechanism in which respiratory tract epithelial cells and dendritic cells sense allergens and respectively evoke immune responses. However, there are sex differences in the behavior of cells, and this leads to differences in pathological conditions.
Allergens that enter the respiratory tract first come in contact with epithelial cells of the respiratory tract. When the epithelial cells sense allergens, they send information through cytokines (signaling substances) to innate immune lymphocytes. Then, innate immune lymphocytes are activated and an immune response occurs. At the same time, dendritic cells—a kind of immune cells—extend their tentacles between epithelial cells to the outside, so they are also in contact with allergens.
Epithelial cells that sense allergens signal dendritic cells to gather and mount the immune response. On receiving the signals, dendritic cells precursors and a type of leucocytes called monocytes are collected from blood vessels, and differentiate into dendritic cells. The existing dendritic cells also join in, and they capture allergens while extending their tentacles and migrate to the lymph nodes. In the lymph nodes, they induce differentiation of T cells, which are agents of the acquired immune response. Then, large number of T cells are activated and return to the lungs in which there are antigens, and this time release an incomparably larger number of cytokines than the innate immune response.
Such enormous quantities of cytokines cause B cells—a type of immune cells—to produce IgE antibodies, eosinophils to be gathered, IgE antibodies to attach to the surface of mast cells, facilitating granules in cells to be secreted. As a result, various symptoms occur such as wheezing, airway obstruction, sputum, and hyperplasia of bronchial smooth muscles. This is the pathogenic sequence of asthma. There are no sex differences in the mechanism in which epithelial cells and dendritic cells are activated in response to allergens.
However, even if there is no difference in the pathogenic sequence, the immune response following allergens’ contact with dendritic cells differed between men and women.” (Dr. Miyasaka)
T cells, which regulate the immune response against allergens, are divided into T helper type 1 (Th1) and type 2 (Th2) cells, and their respective immune responses are called Th1 and Th2 responses. While the Th2 response is mainly related to asthma, it has been reported that in allergic reactions of female patients, T cells that secrete IL-13—a cytokine that induces asthma symptoms—are produced in greater numbers not only compared to individuals without asthma symptoms, but also compared to male asthma patients. Therefore, female patients’ Th2 immune response is stronger than that of male patients.
Femaleness in dendritic cells
Dr. Miyasaka says,
“We investigated the mechanism that promotes the Th2 immune response in female patients. In a study of asthma mice, we found that when mice inhale ovalbumin (OVA) —an allergen—the number of dendritic cells in the lungs and bronchial lymph nodes increases sharply in females (Figure 3).
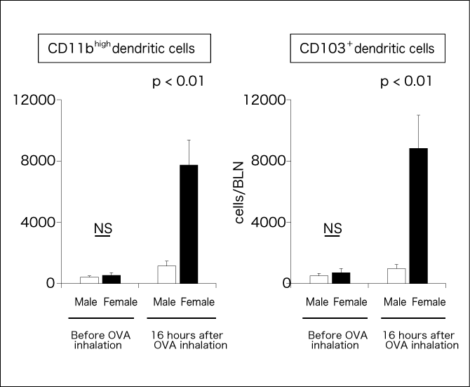
Figure 3. Sex differences in dendritic cells in bronchial lymph nodesThe numbers of dendritic cell subsets—CD11bhigh dendritic cells and CD103+ dendritic cells—were counted in ovalbumin (OVA) inhaling mice. A larger number of these cells denotes stronger inflammation, and the numbers of both cells are apparently higher in females.
Miyasaka, Ohno, et al., The Allergy in Practice, Vol. 38(6), No.514, 55(563)-59(567), 2018.
The number of dendritic cells is the same between male and female mice under normal conditions, but it starts to differ immediately after contact with allergens. In addition, sex differences were found not only in the number but also in the functions of dendritic cells. Females’ CD103+ dendritic cells capture allergens more easily, activate more Th2 cells and increase the number to higher levels than males’, so females’ Th2 cells release a larger quantity of cytokines than males’, therefore the number of eosinophils also increases. Accordingly, asthma symptoms, such as airway obstruction/contraction and mucus production, become stronger in females. So, we can say that females’ dendritic cells have female characteristics (femaleness).
When we further investigated CD11bhigh and CD103+, which are types of dendritic cells, no sex differences were found in CD11bhigh dendritic cells, except for the number of cells. This suggests that the sex differences in the functions of CD103+ dendritic cells are creating the sex differences in the Th2 immune response.
We conducted an experiment to study the relationship between CD103+ dendritic cells and female hormones. We stimulated dendritic cells of asthma mice with OVA in the presence and absence of 17β-estradiol—a type of female hormone. In the presence of 17β-estradiol, the CD86 expression on dendritic cells, which is related to T cell activation, increased, and the quantity of Th2 cytokines produced from the stimulated T cells also increased. This study revealed that the presence of 17β-estradiol in the process of production and activation of dendritic cells is one of the factors that cause femaleness in dendritic cells.”
We came to the result that the behavior of dendritic cells has maleness and femaleness, but when we think about the pathogenic sequence of asthma, we must also look at the immune response of the respiratory tract epithelial cells. Is there maleness and femaleness not only in dendritic cells but also in epithelial cells?
“The immune response in epithelial cells also differed between males and females. In a study of asthma mice, we obtained the result that females’ epithelial cells secrete more cytokines that activate innate immune lymphocytes or cytokines that attract dendritic cells than males’ epithelial cells. Our data shows that there are sex differences in cellular-level reactions from the stage of sensing allergens, even before the immune response. As we are currently in the stage of advancing the study, it is still a hypothesis, but epithelial cells may have sex differences in the process from sensing allergens to signaling dendritic cells to gather and mount the immune response, and this causes sex differences in the pathological conditions of asthma.
On the premise of this hypothesis, if we can identify the molecules that cause the sex differences in the process from when allergen receptors transmit signals to when transcription factors are expressed in the nucleus, we will find a clue to the mechanism of sex differences in the pathological conditions of asthma. We have also formulated a hypothesis that considers the relationship with epigenetic regulation. If we can draw conclusions from these hypotheses, we are likely to be able to find more effective therapeutic methods for female asthma patients, and prevent aggravation associated with sex differences. We are pursuing research with the aim of contributing to a more effective therapy.” (Dr. Miyasaka)
We discussed at the beginning that, as the reversal of the asthma prevalence in men and women after adolescence is a tendency observed in many countries, sex differences may be attributable to factors other than the environment or diet, and that the effect of sex hormones may be relevant. However, we understood from the research by Dr. Miyasaka’s group that sex differences in cells themselves affect the pathological conditions of asthma. Then, to what extent has the effect of sex hormones been clarified?
Maleness and femaleness in immunity
There is a report on the extent to which female hormones (estrogen, progesterone) and male hormones (androgen) affect the immune system, and how. Estradiol—a type of estrogen—promotes activation of dendritic cells (DCs) and production of chemokines (CCL2) that attract dendritic cells, and also increases the expression level of Toll-like receptors that activate the innate immune response. Activation of dendritic cells and induction of chemokines that attract monocytes play important roles in asthma, and they are affected by estrogen.
There is also a report that progesterone has a suppressing effect, while the effect of male hormones on dendritic cells is still unknown.
“Although female hormones are presumed to promote the immune response, the secretion levels of estrogen and progesterone respectively increase or decrease in the menstrual cycle and they overlap each other, so there must be a period when the immune response is activated and a period when it is suppressed. Pathological conditions consist of an interdependence of hormones and immunity. In the case of men, maleness of immunity is said to develop in the fetal stage as male hormone exposure starts in line with genitalia development from around the 10th week of pregnancy and is reflected in the amount of IgE antibodies.
Over a lifetime, women experience a sudden decrease in female hormones after menopause, and this is also considered to be a factor in sex differences. Both in women and men, the effect of sex hormones diminishes toward the end of the lifetime, but the effect of sex chromosomes on immunity remains. So, human immunity is known to be affected by sex in all phases of life from fetus to the end of lifetime.
My understanding is that there are maleness and femaleness in immunity, both of which act positively or negatively depending on the disease. The sex of cells themselves, which forms the pathological conditions of diseases associated with the immune response, is affected by various environmental and genetic factors, as well as sex hormones. Basic research for explaining these is needed.” (Dr. Miyasaka)
Sex differences in pathological conditions appear in various phases over our lifetime. Basic research on sex differences has much contribution to make for establishing effective therapies to address such differences.