Almost all creatures have blood, but not all creatures’ blood is red. The red color of our blood results from the iron in the hemoglobin of red blood cells binding with oxygen. Oxygen is carried through our bodies via this process. Blood also fulfills numerous other roles essential to life, such as transporting nutrients and hormones, and recovering carbon dioxide, lactic acid, and other waste products. In addition, blood is equipped with outstanding defensive mechanisms that maintain its stable circulation; a particularly important example of this is hemostasis, which is directly involved in life-threatening situations. Blood is one of the organs fundamental to sustaining life.
Special Feature 1 – The Mechanisms and Functions of Blood Learning about the diverse functions fundamental to sustaining life
composition by Yuko Watanabe
Blood flows through blood vessels throughout the body, reaching every tissue in the body. Blood plays an important role in sustaining life, by transporting oxygen, nutrients, and hormones, collecting waste products such as carbon dioxide and lactic acid, and protecting against infection from foreign substances such as bacteria and viruses, and stopping bleeding.
Red blood cells make up 99% of the cellular components of blood
Our bodies contain around 4-5l of blood, accounting for approximately 8% of our body weight; about 90% of this blood circulates throughout the body, while the remaining 10% is stored in the liver and spleen. If a blood sample is collected and centrifuged after the addition of an anticoagulant to prevent clotting, the blood separates into three layers. These consist of a liquid component and cellular components (blood cell components). The uppermost layer of thin, yellowish fluid is the liquid component, which is called plasma and accounts for about 55% of blood. Below this is a whitish layer consisting of white blood cells and platelets, while the red layer underneath it consists of red blood cells; together, these three types of cellular components account for around 45% of blood (Figure 1).
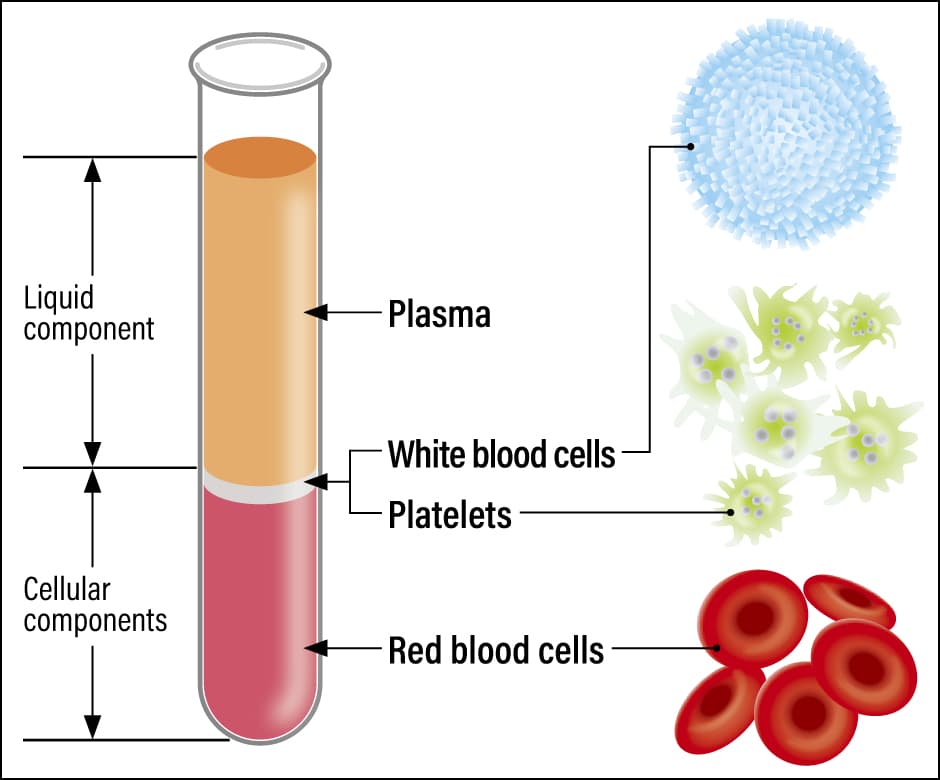
Figure 1. Blood components and their rolesBlood is composed of a liquid component called plasma, and cellular components in the form of white blood cells, platelets, and red blood cells. There are five types of white blood cells (neutrophils, lymphocytes, monocytes, eosinophils, and basophilic leukocytes), which are responsible for immunity and work together to protect the body.
Since four pages are far from enough to explain everything about the liquid and cellular components of blood, I would like to provide an overview in the first half of this article, and then use the second half to explain the outstanding mechanism called hemostasis, which is one of my specialties.
●Plasma
Plasma is more than 90% water, about 7% protein, and the rest lipids, carbohydrates, amino acids, inorganic salts, metals, hormones, and electrolytes.
The main protein substances are albumin and globulin. Other proteins include coagulation factors, which promote blood clotting, and coagulation factor inhibitors, which inhibit their action; and also fibrinolytic factors, which dissolve blood clots (thrombi) in a process called fibrinolysis, and antifibrinolytic factors, which inhibit their action. The coagulation and fibrinolytic systems support the smooth flow of blood by maintaining a delicate balance between these factors so that none of these factors predominates over the others.
●Red blood cells
Red blood cells account for 99% of the cellular components of blood, and there are estimated to be around 5 million cells in 1 µl (0.001 ml) of blood. Also known as erythrocytes, these cells are disk-shaped, 7-8µm (0.007-0.008 mm) in diameter and about 2µm thick, with depressions on both sides.
Red blood cells contain a large quantity of the protein hemoglobin. An iron-containing component of hemoglobin called heme binds with oxygen and transports it to tissues throughout the body. As their cell membranes are highly flexible and resilient, red blood cells can change shape to pass through even very small blood vessels, such as capillaries, thereby ensuring that oxygen reaches every part of our tissues.
The lifespan of a red blood cell is about 120 days. Old red blood cells are destroyed in the spleen or other organs and phagocytosed by macrophages. The released hemoglobin is reused both for the protein portion, globin, as amino acids, and for the heme portion, iron, for new hemoglobin synthesis.
●White blood cells
There are between 5,000 and 10,000 white blood cells in 1 µl of blood. Also known as leukocytes, these cells are responsible for controlling infection (immunity) by protecting the body against foreign substances such as bacteria and viruses. There are five types of white blood cells —— neutrophils, eosinophils, basophils, monocytes, and lymphocytes —— all of which work together. Some cells perform a variety of immune-regulating functions: for example, some engage in phagocytosis by engulfing and consuming foreign substances, while others present information about the foreign substances to other white blood cells, release chemical substances to attack, or suppress and provoke allergic reactions.
●Platelets
Platelets are the smallest cellular components of blood, oval-shaped cells 2-4µm in diameter, with 150,000-300,000 cells in 1 µl. Platelets play a part in hemostasis and blood vessel repair.
When a blood vessel is damaged, platelets gather at the site of the injury and bind (adhere) to the collagen in subcutaneous tissue with the aid of von Willebrand factor (VWF). When a stimulus enters the platelets adhering to the area, they change shape from disk-shaped to spherical and form platelet clumps. In addition, granules inside the platelets release platelet-activating substances, which cause the formation of a more stable platelet thrombus (primary thrombus), thereby plugging the injury to the blood vessel (Figure 2).
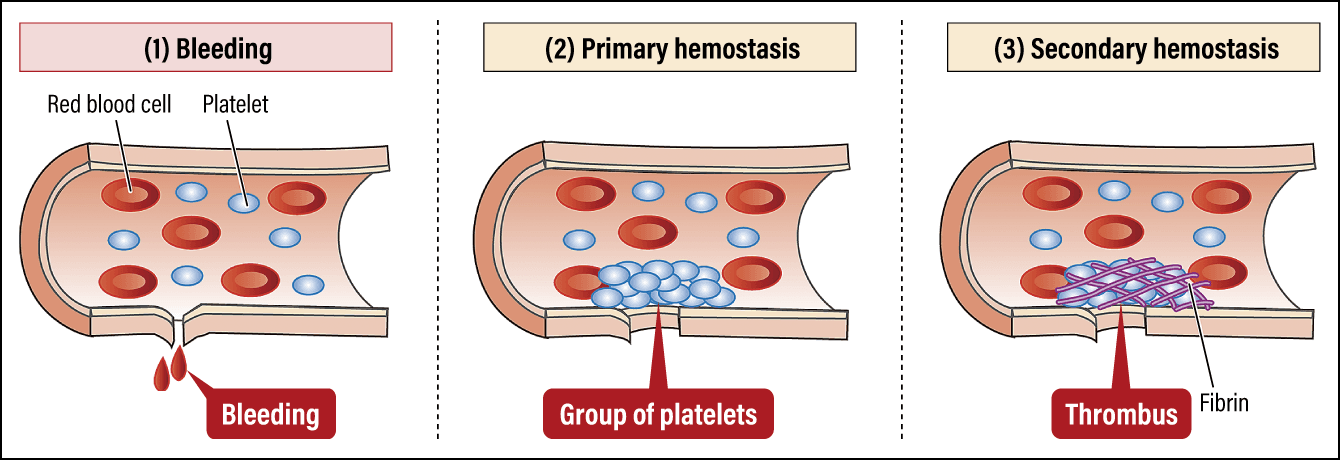
Figure 2. Platelet functions and pathologyWhen bleeding from a blood vessel occurs, the hemostasis mechanism immediately mobilizes, smoothly carrying out primary and secondary hemostasis to seal the wound. Once hemostasis is complete, the mechanism that dissolves thrombi gets to work to restore the original blood flow.
The vascular systems of mammals and other vertebrates are closed circulatory systems, in which blood is sent from the heart through the arteries to the capillaries, and then returns to the heart via the veins. As such, the blood circulates via a completely closed route, with no exit. Incidentally, an open circulatory system is a mechanism in which there are no capillaries linking arteries to veins; instead, blood that has flowed through the arteries circulates by flowing between tissues and returning either to the veins or directly to the heart. Insects and non-cephalopod mollusks such as snails have an open circulatory system.
In a closed circulatory system, an injury somewhere in a blood vessel that causes blood to keep flowing out or blockage of the blood flow due to blood having clotted in a blood vessel poses a risk to life, due to a decrease in or interruption of the flow of blood to vital organs. The system is equipped with several outstanding defensive mechanisms to maintain a stable circulation of blood flow.
In primary hemostasis, the action of the platelets forms a thrombus at the opening of the wound in the blood vessel. As this thrombus is the equivalent of a band aid, it will peel away easily when exposed to the powerful force of the blood flow. Accordingly, the plasma protein fibrinogen is converted into a fibrous substance called fibrin that covers the primary thrombus to reinforce it, forming a secondary thrombus (fibrin clot) that consolidates hemostasis. Repair of the blood vessel takes place at the same time, with vascular endothelial cells proliferating around the site of the injury and covering it with new cells.
Once bleeding is completely stopped, the next step is to dissolve the thrombus blocking blood flow. Through this phenomenon, which is called fibrinolysis, the thrombus is ultimately dissolved, the blood vessel is repaired, and the blood flow returns to normal.
The formation of the secondary thrombus from the primary thrombus is promoted by proteins in plasma called coagulation factors, of which there are nearly 10 different types. The reaction of these factors is rapid and once switched on, it is amplified like a rushing waterfall, hence the name “coagulation cascade” (waterfall).
This kind of coagulation reaction in the blood is a hemostasis mechanism triggered by the leakage of blood from a blood vessel and is called physiological hemostasis. Impairment of this physiological hemostasis mechanism causes bleeding disorders, the leading example of which is hemophilia.
The blood that flows through our blood vessels ordinarily needs to be kept fluid, so that it can carry oxygen and nutrients to every part of the body. Vascular endothelial cells play an important role in this process. As vascular endothelial cells have functions that prevent blood from coagulating on the cell surface and dissolve thrombi, they work constantly to ensure blood does not clot easily.
The three factors that promote thrombus formation
Our blood is fully equipped with hemostatic system functions to protect us from the life-threatening risk of bleeding. These may have been essential for primitive man, who sustained many injuries involving major bleeding, but for people today, these safeguards have become excessive and have resulted in a situation in which thrombi can easily form in blood vessels. In contrast to “physiological hemostasis, ”a situation in which blood clots in the blood vessels in this way is called “pathological hemostasis.” When a thrombus gets blocked in the arteries of the heart or brain, myocardial infarction or cerebral infarction can occur. If a thrombus forms in a vein in the leg and goes on to block a blood vessel in the lungs, pulmonary embolism (economy class syndrome) can occur.
Three major factors contribute to the formation of blood clots (Figure 3). The first is vascular endothelial cell injury. When blood vessels are injured as a result of surgery, trauma, fracture, or intravascular catheter insertion, for example, the ability to prevent thrombus formation is impaired.
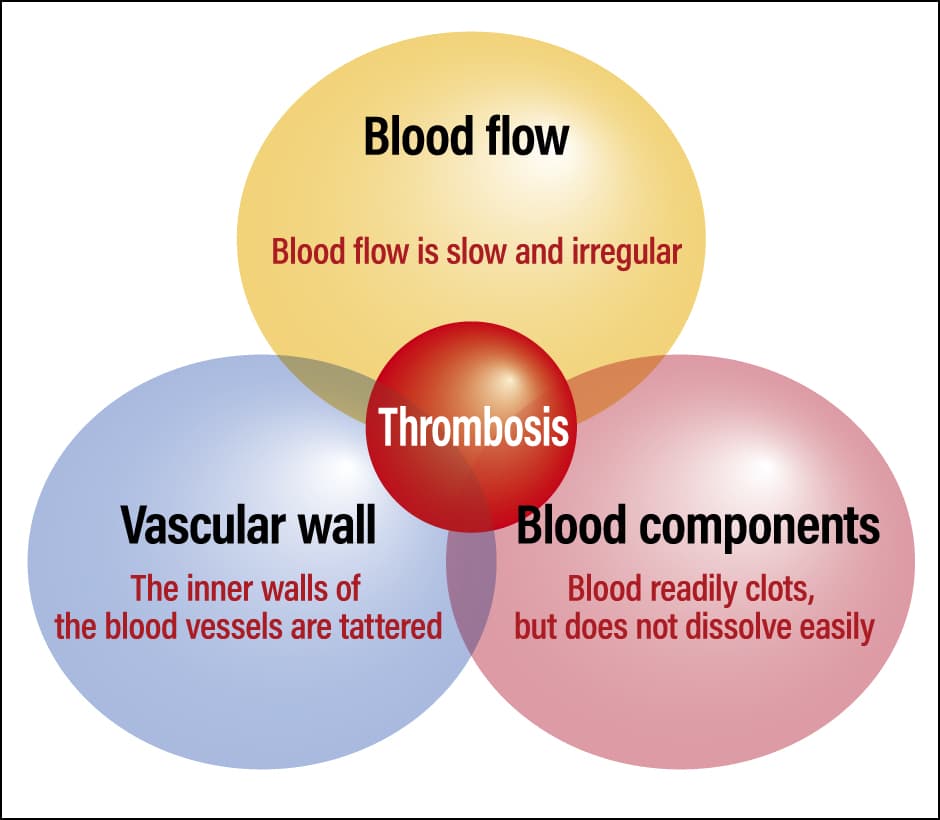
Figure 3. The three factors promoting thrombosisThe three factors promoting thrombosis (Virchow’s triad), which were first propounded in the mid-19th century by a German pathologist called Rudolf Virchow. They are still regarded as important today, on the grounds that the risk of thrombosis is elevated if these three factors overlap.
The second is stasis of blood flow. Thrombi form more readily when blood pools or its flow is disrupted. Bedridden, long-distance travel, obesity, pregnancy, and heart or lung disease increase the risk of blood stagnation. We are concerned about pulmonary embolism (economy class syndrome) among those who are spending time in evacuation centers or staying in cars due to the 2024 Noto Peninsula earthquake.
The third factor is changes in blood properties. In severe infections, procoagulant factors are expressed on the surface of white blood cells in response to stimulus from inflammatory cytokines and the like, or factors that activate coagulation are secreted from cancer cells, giving rise to changes in blood properties that result in a greater tendency toward thrombus formation. Additionally, dehydration causes blood to become more concentrated, making it sticky and easier for thrombi to form.
In health-related topics, it is often said, “Thin blood is prone to clotting, so let’s make it thinner.” In today’s age of food saturation, diabetes and hyperlipidemia promote arteriosclerosis, which causes blood vessels to harden, in addition, our blood tends to clot easily because it has an excessive amount of hemostatic function by nature. That is why I would like readers to understand the importance of keeping blood thinner.
Erythrocytosis (also known as polycythemia), which involves an increase in the concentration of red blood cells, causes blood to become more viscous, promoting the formation of thrombi. The causes of erythrocytosis are varied: while polycythemia vera is a blood disease, erythrocytosis arising from causes other than a disease is called secondary erythrocytosis. Red blood cell concentrations can also increase as a result of lifestyle-related factors, in the form of dehydration, excessive alcohol consumption and smoking, obesity, and stress. An increase in white blood cells indicates that the body is suffering an infection somewhere, and can also arise due to gingivitis (gum disease), chronic sinusitis, and even excessive smoking.
Thus, lifestyle factors affect blood composition, but making improvements will restore its normal composition. I would advise readers to review their day-to-day lifestyles and think about whether their blood is healthy.
Research into artificial red blood cells and platelets is advancing
Talking about blood, the decline in the number of young blood donors has become a problem both within Japan and overseas. When I ask students in my lectures about their blood donation experience, it used to be the case that around half would have done so, but these days it is virtually none. According to statistics released by the Ministry of Health, Labor and Welfare based on a survey by the Japanese Red Cross Society, the number of young people donating blood has declined significantly, and it has become clear that the volume of blood donated is supported by donations from middle-aged and elderly people aged 40 to 69 (Figure 4). If things continue at this pace, it will soon become difficult to procure a stable supply of blood.
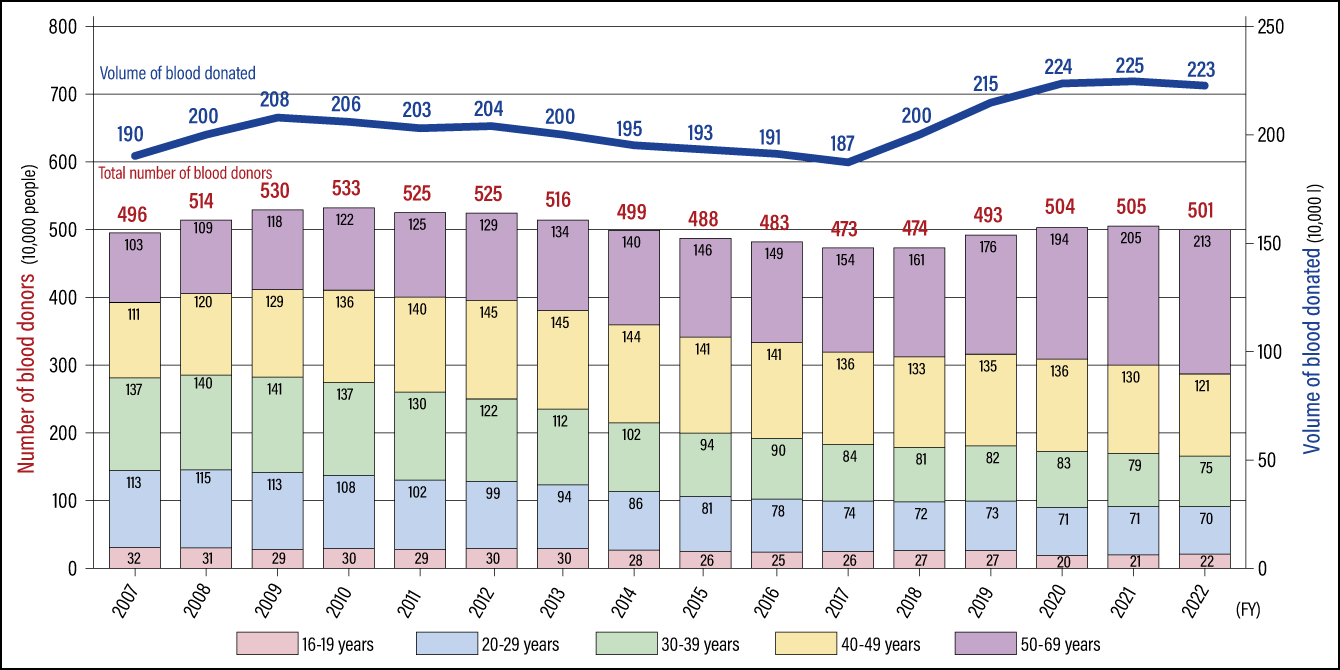 * Since FY2018, figures for the volume of blood donated are based on the total volume of blood donated, including both whole blood and blood donated via apheresis (donation of specific blood components) as calculated at the manufacturing stage (including the volume of blood preservation solution).
* Since FY2018, figures for the volume of blood donated are based on the total volume of blood donated, including both whole blood and blood donated via apheresis (donation of specific blood components) as calculated at the manufacturing stage (including the volume of blood preservation solution).(Modified version of chart produced by the MHLW from materials submitted by the Japanese Red Cross Society)
Figure 4. Number of blood donors by age and trends in blood donation volumesWhile figures for the number of blood donors and the volume of blood donated have been on the rise in recent years, the number of blood donors aged in their teens to 30s has seen a fall of around 30% in the last decade and the share of the younger generation among all blood donors has also declined. There are also concerns about a decline in the younger generation’s awareness of blood donation, so challenges include increasing understanding of blood donation and creating opportunities to donate blood, in order to secure a stable supply.
About half of donated blood is used for blood transfusions and other therapeutic purposes, while the remainder is used to make blood products. A decline in the ability to secure blood via donation would have serious implications, making it impossible to perform surgery requiring blood transfusions or to make the blood products required for treatments.
If we could not secure blood via donation, we must rely on artificial blood components. Research into artificial red blood cells and platelets is progressing, with a research team at Kyoto University working on platelets derived from iPS cells, while a research team from Keio University is working on platelets derived from adipose tissue. Some studies have already reached the clinical trial stage, and the clinical application of these cells is anxiously awaited.
Medical personnel regard the use of artificial blood components as a form of blood replacement therapy, but unlike organ transplantation, these components do not survive and function in the patient’s body for their entire lifetime. The lifespan of a red blood cell is around 120 days, while platelets last only about 10 days, so temporary supplementation would ensure adequate functions, as long as there were no acute side effects or complications. We understand that it is very difficult to create by artificial means something equipped with as many incredible functions as human blood, but we have high hopes for the creation of artificial blood.
You never know when you might suffer an injury or illness necessitating surgery that requires a large blood transfusion. I would like readers to donate blood, not only for their own sake, but also for that of countless other people.