Oligosaccharides are known to be a low-energy sweetener offering the same degree of sweetness as sugar. In addition, they are equipped with a diverse array of functions, one of which is regulating the condition of the gut by serving as food for beneficial gut bacteria such as Lactobacillus and Bifidobacterium. However, recent studies have shown that not only “good” bacteria, but also other gut bacteria can feed off some of them. Accordingly, attention has turned to oligosaccharides that promote the proliferation of beneficial bacteria alone, and development efforts in this area are progressing. Scientists say that in the future, it will be possible to use oligosaccharides to create next-generation prebiotics readily capable of improving the gut microbiota.
Special Feature 1 – Focus on Sugars Developing new oligosaccharides that boost only beneficial gut bacteria
composition by Toshiko Mogi
As a glycoscientist, I am involved in research focused on creating functional oligosaccharides that are useful in maintaining and improving human health. In this article, I would like to introduce oligosaccharides and their functions.
Oligosaccharides are composed of 2-10 monosaccharide units.
The first thing I want to tell you is that numerous carbohydrates exist in the natural world in various forms. Via photosynthesis, plants produce starch to serve as a source of energy. We humans, too, consume carbohydrates in our meals and store it in the muscles and liver in the form of glycogen. Cellulose in wood, from which paper is made, is also a carbohydrate made up of a chain of glucose molecules. The principal component of konjac (Amorphophallus konjac) tubers, glucomannan, is a carbohydrate, as well. The main component of the exoskeletons (shells) of insects and of shrimps, crabs, and other crustaceans is chitin, which is actually a polymer of monosaccharide called N-acetylglucosamine. Chitin is also found in mollusks such as squid and in mushrooms and other mycophyta. Thus, many different carbohydrates can be found in the natural world in a variety of forms. But which are oligosaccharides and why are they called that?
Classifying carbohydrates by molecular structure, the smallest units are called monosaccharides, which include glucose and fructose. The carbohydrates composed of 2-10 monosaccharides are called oligosaccharides. “Oligo” is a word derived from Greek that means “few.” Also classed as oligosaccharides are disaccharides, which consist of two monosaccharide molecules. Disaccharides include sucrose (table sugar, which is composed of glucose and fructose); maltose (consisting of two glucose molecules), which is the principal component in starch syrup; and lactose, which gives milk its sweetness. Additionally, there are megalosaccharides and polysaccharides, which are composed of 10-100 monosaccharides and more than 100 monosaccharides, respectively. Thus, monosaccharides polymerize into longer chains, and the names by which we call these carbohydrates vary accordingly (Figure 1). The carbohydrates have some “hands” (hydroxyl groups), which means that the way they polymerize varies according to which hand is linked to which. Given that more than 100 monosaccharides have been discovered to date, you can probably imagine just how vast a range of carbohydrates can be formed, based on the variety of possible bonding patterns.
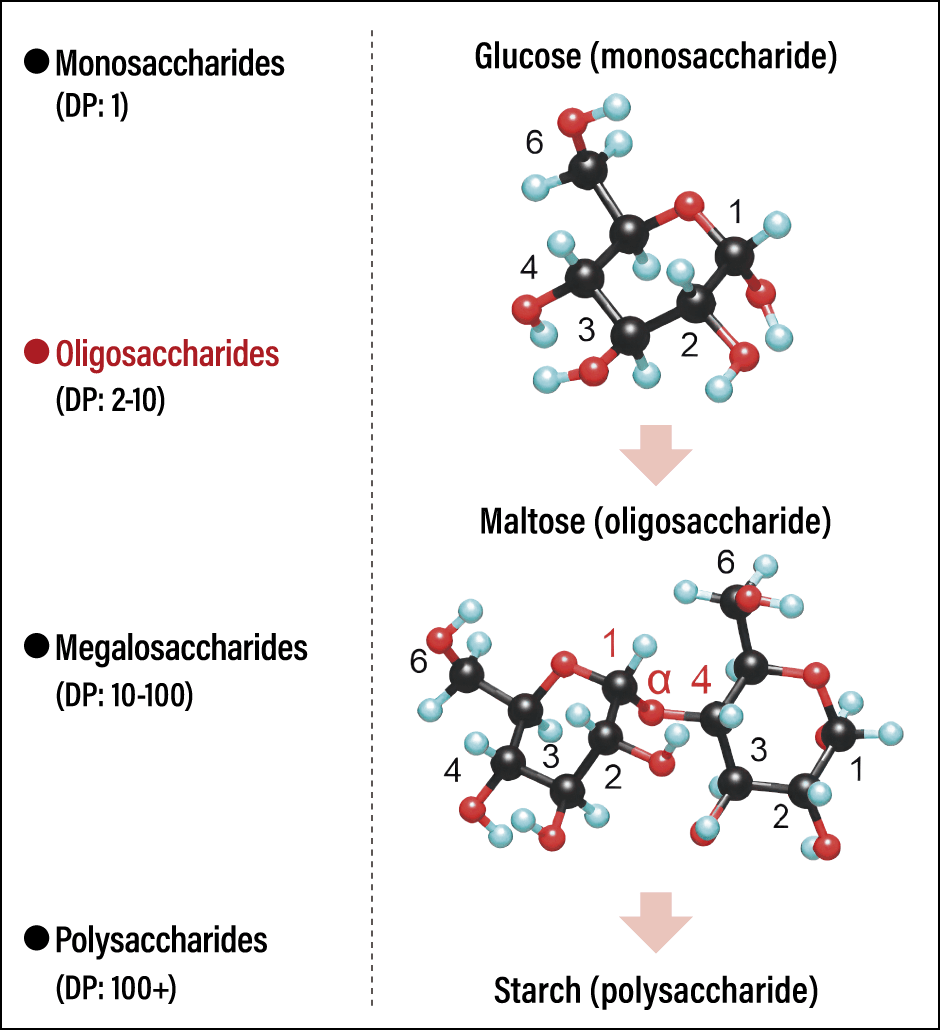
Figure 1. Types of carbohydrate and examples of their polymersCarbohydrates are classified from monosaccharides to polysaccharides, based on their degree of polymerization (DP). The right-hand side shows examples of monosaccharide, oligosaccharide, and polysaccharide polymers.
Incidentally, the sucrose used in daily life as a sweetener is an oligosaccharide that is crystallized after being extracted from sugarcane (Saccharum officinarum) or sugar beet (Beta vulgaris). Numerous other oligosaccharides also exist in the natural world, but despite their abundance, not many have been subject to the evaluation of their functionality or taste. In other words, only a very few oligosaccharides can be used scientifically or industrially, so the reality at present is that we are not fully leveraging their potential. To ensure that oligosaccharides can readily be used, we need to find safe ways to mass-produce them and supply them to consumers at a reasonable price. Therefore, I believe that creating oligosaccharides enzymologically would be worthwhile.
Oligosaccharides have a diverse array of functions, which can be broadly classified into three categories. Primary functions are their nutritional properties as food, such as their ease or difficulty of digestion and their energy value; secondary functions are their physicochemical characteristics, such as moisture retention capabilities; and tertiary functions are their bioregulatory functions, such as gut regulation and anticariogenic properties.
Amid rising health consciousness in recent years, there has been a growing focus on the bioregulatory functions of oligosaccharides. Some time ago, I investigated the prevalence of foods associated with functional oligosaccharides and found they accounted for around 40% of all food for specified health uses (FOSHU). This figure may well have increased by now.
The function of modifying gastrointestinal conditions
The first FOSHU that touted gut regulation functions was a fructo-oligosaccharide developed by a Japanese food manufacturer. Formed from 2-4 fructose molecules bound to a glucose molecule, fructo-oligosaccharides offer a level of sweetness close to that of table sugar. While they are found in fruit and vegetables such as onions and burdock root, they cannot be extracted in sufficient quantities for mass production and are costly. Accordingly, the food manufacturer created a fructo-oligosaccharide from table sugar —— which consists of one glucose molecule bound to a single fructose molecule —— by enzymologically polymerizing the fructose. Fructo-oligosaccharides produced in this way were permitted to display the functional indication “food to modify gastrointestinal conditions.” This was in 1993, exactly 30 years ago. In fact, this phrase “modify gastrointestinal conditions” also holds the key to understanding functional oligosaccharides.
A vast array of oligosaccharides can be found in the natural world; the ones used as FOSHU are non-digestible oligosaccharides. Non-digestible means that they cannot readily be broken down by human digestive enzymes and therefore cannot easily be used as a source of energy. Most carbohydrates that we ingest orally are broken down by digestive enzymes secreted by our digestive organs and, once absorbed, are used as energy as they travel around the body. In contrast, non-digestible oligosaccharides are highly resistant to digestion, enabling them to reach the gut, where they can become food for gut bacteria.
Gut bacteria can be broadly classified into three types: beneficial bacteria that have a positive effect on the body, such as Bifidobacterium and Lactobacillus; harmful bacteria, which have a negative effect; and neutral bacteria, whose role is often unclear. In a healthy human gut, beneficial bacteria are known to inhibit the establishment and proliferation of harmful bacteria, thereby helping to maintain and improve health. In other words, the bioregulatory function of oligosaccharides —— namely, their mechanism for modifying gastrointestinal conditions —— improves the gut microbiota by providing food for beneficial bacteria in the gut, which has a positive effect on health.
However, it is not only beneficial bacteria for which oligosaccharides provide food. Harmful and neutral bacteria can also feed on some of them. It goes without saying that harmful bacteria have undesirable effects, but in recent years, it has become apparent that some neutral bacteria can have an adverse impact on humans. Accordingly, when humans consume food for beneficial bacteria, it is desirable to ensure that it is of a kind that avoids the proliferation of normal gut flora that have negative effects, including both harmful bacteria and neutral bacteria.
To maintain and improve health in an efficient way, we should aim to selectively increase only the number of beneficial bacteria by consuming oligosaccharides that only beneficial bacteria feed on. It was this goal that inspired the idea of a functional oligosaccharide aimed at bringing about the proliferation of specific beneficial bacteria —— in other words, next-generation prebiotics.
Various prebiotic foods based on oligosaccharides are already on the market. However, when our research team investigated the functions of them, we found that, unfortunately, they did not bring about an increase solely in specific beneficial bacteria with pinpoint accuracy. We discovered that, at the very least, they served as food for the other gut microbiota as well. Against this backdrop, our research team is developing next-generation prebiotics capable of selectively bringing about the proliferation of specific beneficial bacteria alone.
Using existing carbohydrates to create beneficial oligosaccharides
As I stated at the beginning, I am a glycoscientist who studies the development of oligosaccharides that are beneficial in maintaining and improving human health. A large, diverse array of oligosaccharides can be found in the natural world, but as it is difficult to achieve their mass preparation at low cost, the reality at present is that only a very few oligosaccharides can be used scientifically and industrially. Accordingly, we turned the idea around and decided to try to produce beneficial oligosaccharides by using existing carbohydrates and altering their structure.
As I stated earlier, carbohydrates have some “hands,” which means that the type of bond between them varies according to which hand is linked to which. What we are trying to do is to produce functional oligosaccharides by severing the joined hands of existing carbohydrates found in the natural world and then joining them in a different pattern. In addition, we focused on oligosaccharide synthesis reactions using carbohydrate phosphorylases (carbohydrate-processing phosphorolytic enzymes) to serve as the scissors and glue in this process.
The carbohydrate phosphorylases are safe natural catalysts that are involved in glycometabolism in a variety of organisms. The enzymatic reactions are often likened to the relationship between a key and a keyhole. This means that an enzyme (the keyhole) will react only with a certain substance (the key). One feature of phosphorylases is that they produce only oligosaccharides that have a certain bond. However, when we first embarked on the research, there were only a dozen or so known phosphorylases, so, in order to produce numerous types of oligosaccharides, we needed to search for as-yet-unknown phosphorylases that would provide keyholes that matched them perfectly. We therefore tapped into bioinformatic techniques and methods for screening the microorganisms that produce those enzymes, and have already discovered a large number of new phosphorylases.
In parallel with our research into discovering new phosphorylases, we pursued studies aimed at developing technology for manufacturing oligosaccharides that had hitherto been unfeasible to mass produce. As a result, we developed a technology for low-cost mass production that achieves high-yield conversion of cheaply obtained carbohydrates abundantly available in the natural world into high value-added functional oligosaccharides (Figure 2).
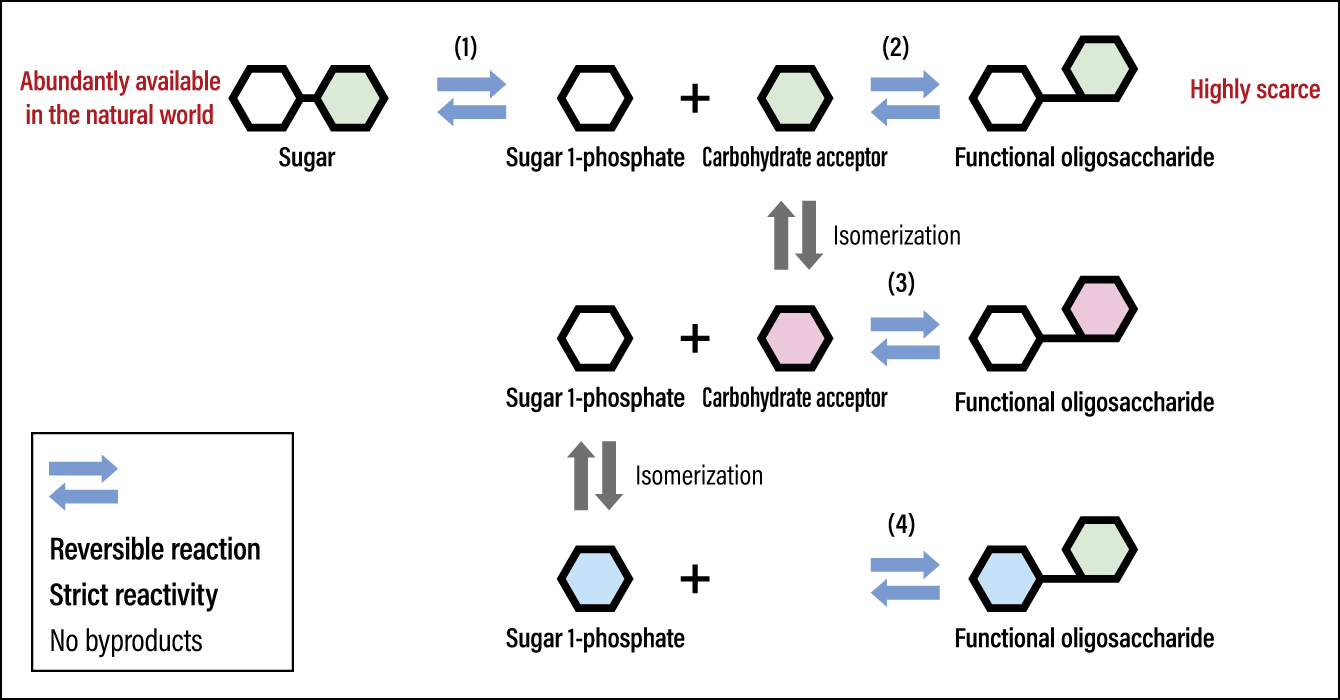
The oligosaccharides produced using this technology that we developed already numbers in excess of 300. Working day-to-day with the students in my laboratory, we strive to produce new oligosaccharides and have created a storage bank for them at the laboratory. Focusing on the large, diverse array of oligosaccharides in the bank, we are also building an oligosaccharide library, which contains the findings from our investigation of the correlation between structure and function for each and every item in the database. As a part of the research, we investigated the ability of the diverse oligosaccharides in the bank to improve the gut microbiota, containing beneficial bacteria, and harmful bacteria from the human gut. As a result, we have recently discovered an galacto-oligosaccharide that selectively promotes the proliferation of the beneficial bacterium Bifidobacterium alone, and have also identified the gene required for bifidobacteria to grow using this specific galacto-oligosaccharide as a source of nutrition.
Foods like oligosaccharides that can feed beneficial bacteria in the gut are called prebiotics, while living microorganisms such as Bifidobacterium and Lactobacillus that have a helpful effect when consumed in appropriate amounts are called probiotics. In recent years, a method of improving the gut environment called synbiotics, which combines prebiotics and probiotics, has been proposed.
Accordingly, we combined our recently discovered galacto-oligosaccharide with bifidobacteria and proved that they substantially inhibited the growth of Clostridium difficile —— the bacterium that causes pseudomembranous colitis —— and considerably reduced the quantity of toxin produced by these bacteria, as well as reducing weight loss in pseudomembranous colitis model mice. Sometimes, the administration of antibiotics destroys the balance of the normal gut microbiota, and the process of microbial substitution, in which a particular bacterial species increases to abnormal levels, causes inflammation in the colon (infectious colitis). Pseudomembranous colitis is a type of infectious colitis that occurs when C. difficile proliferates to an abnormal degree. It would be fair to say the combination of the galacto-oligosaccharide that we discovered with bifidobacteria demonstrated the potential for drug discovery and the development of new therapies for pseudomembranous colitis using next-generation prebiotics.
Our research team coined the term “next-generation prebiotics” to describe oligosaccharides with the function of bringing about the proliferation of only beneficial bacteria that help to maintain and improve human health, like the galacto-oligosaccharide we recently discovered. The phrase “next-generation” expresses hopes and expectations for the future. It also encompasses the connotation of something more advanced and with a higher level of function than the prebiotics currently available. Nevertheless, our intention is not to repudiate existing prebiotics. It simply means that our research has resulted in the creation of more advanced prebiotics than the ones currently available.
Prebiotics tailored to the individual gut environment
In my laboratory’s oligosaccharide library, there are many newly created oligosaccharides in addition to the galacto-oligosaccharide that we have discovered. The oligosaccharide about which we published an article had the function of bringing about the proliferation of only bifidobacteria, but there are also oligosaccharides capable of selectively causing the proliferation of other beneficial bacteria. Little by little, we are gradually shedding light on which oligosaccharides with which structures and bonds increase the number of which bacteria.
In addition, according to recent research reports, it is becoming apparent that there is a relationship between human diseases and the gut microbiota. Investigations of the gut environments of humans have found bacterial species present in higher or lower numbers or proportions, depending on the disease or symptom. This would suggest that, in order to maintain and improve health, we need to deliver to the gut oligosaccharides that promote the proliferation of specific bacteria that will improve the gut environment of each particular individual. Furthermore, until now, regulating the gut environment has been virtually synonymous with promoting the proliferation of Lactobacillus and Bifidobacterium, but in future, the spotlight might well fall on different human gut bacteria and the way in which they function. There is also a possibility that selectively promoting or inhibiting the proliferation of gut bacteria that demonstrate distinctive changes in cases of diabetes or obesity could treat those conditions. In fact, we have provided items from our oligosaccharide library to experts in various fields, and are in the process of discovering oligosaccharides that promote the proliferation of specific bacteria and have functions that are helpful in maintaining and improving human health.
Hopes are high that the future might see the advent of an age in which it is possible to provide prebiotics tailored to each individual’s gut environment and to readily improve the gut microflora, which varies from person to person. Our research team therefore calls this concept “tailor-made prebiotics.” If we could produce oligosaccharides with advanced functions cheaply and in large quantities, and have food manufacturers use such oligosaccharides as raw materials, we might well be able to provide society with products containing tailor-made prebiotics —— which could also be described as next-generation synbiotics —— that combine the bacteria whose proliferation we wish to promote with the oligosaccharides on which they feed.
However, before obtaining approval of the authorities, a huge amount of time and money will be required for checks of their safety and other features. I do not know when this will actually happen, but I am working on the creation of new oligosaccharides at my laboratory, in the hope that one day, we will be able to provide consumers with reasonably priced oligosaccharides with advanced functions and foods made from them, and thereby maintain and improve their health.