Genome editing is a technology for manipulating genes by specifically cutting a target DNA sequence with the aid of a DNA cleavage enzyme. Unlike genetic recombination , which involves inserting exogenous genes, genome editing is regarded as a comparatively safe means of modifying an organism’s genes and has become prevalent in a variety of fields. What has made this advance possible is an innovative genome editing tool called CRISPR-Cas9. The scientists who developed it were awarded the Nobel Prize in Chemistry in 2020. This tool has now opened up a wide range of possibilities for “manufacturing” that would be impossible without genome editing technology.
Special Feature 1 – The Truth about Genome Editing The diverse “manufacturing” possibilities presented by innovative tools
composition by Yuko Watanabe
illustration by Rokuhisa Chino
Genome editing technology emerged in the latter half of the 1990s as an alternative to conventional genetic recombination technology. My specialism is developmental biology. I had been using sea urchins as model organisms in my research investigating the cell differentiation process in living ova. Around 2007, I began to focus on zinc finger nucleases (ZFNs) —— part of the first generation of genome editing technology —— as a tool for tracking changes in the level of expression of genes in sea urchin embryos. As commissioning the production of ZFNs was expensive, I worked on producing them myself and, having been successful after two or three years, published an article in 2010.
That same year saw the release of TALENs, the second-generation tool, which are easier to modify than ZFNs. Our research team developed TALENs of our own that demonstrated higher activity and were convenient to use, which we called Platinum TALENs. We also developed a production system, enabling the genes of a variety of organisms to be modified, and it is now used in fish breeding and even medical care.
Around this time, we began creating a community of researchers for those with an interest in genome editing technology or who wish to learn how to use the technology at Hiroshima University. Through this community, we shared the latest genome editing technologies and engaged in lively discussion of social acceptance of genome editing, as well as pursuing and strengthening collaboration with scientists overseas. The community evolved into the Japanese Society for Genome Editing, which was founded in 2016. I served three terms as its president, before handing over the reins to the current president, Professor Tomoji Mashimo of the Institute of Medical Science, the University of Tokyo, after six years.
Researchers are developing their own genome editing tools
In 2012 came the third-generation tool, CRISPR-Cas9. This innovative gene modification tool is not only easy to handle, but also able to knock out gene functions with pinpoint accuracy. Easy to use even for those who are not experts, CRISPR-Cas9 has facilitated tremendous advances in life science research; it has been used for research and development in a variety of fields, including not only basic research, but also agriculture, drug discovery and medical treatment, biomanufacturing, and the environment.
CRISPR-Cas9 remains an essential tool in basic research even now. It is licensed for an affordable fee, so that anyone in the world can readily use it, as long as it is for the purpose of basic research, and that commitment is still upheld today.
However, patent rights involving intellectual property produced using CRISPR-Cas9 are complex; if a company seeks to use the tool for industrial purposes to make a profit, it must pay an expensive patent royalty. For example, the use of CRISPR-Cas9 in the genetic modification of cells in order to develop a pharmaceutical reportedly incurs a royalty in excess of ¥10 billion, which presents an obstacle to its industrial use.
At research institutes in Japan and overseas alike, scientists are developing their own genome editing tools. However, as we have reached the stage at which it is becoming impossible to generate distinctive features without a technology that far surpasses CRISPR-Cas9, these tools will not win out amid fierce competition. Nevertheless, information about upgraded versions of CRISPR-Cas9 and other genome editing technologies are published online on a virtually daily basis, providing a sense of the speed with which genome editing technology is being developed and the scale of its influence.
Although CRISPR-Cas9 is an outstanding technology, it is not foolproof and can have off-target effects, in which a sequence similar to the target sequence is cut by mistake and a mutation introduced in an unexpected location. Given this drawback, there are times when TALENs are better —— for instance, the Platinum TALENs we developed at Hiroshima University make it possible to avoid off-target effects as well. I believe that the most effective approach is to determine usage according to the situation, using CRISPR-Cas9 for basic research, and ZFNs or TALENs for industrial purposes, as they are cheaper and entail no concerns about expensive patent royalties.
At Hiroshima University, we have changed direction and moved away from pursuing the development of a genome editing tool that will surpass CRISPR-Cas9. Instead, we are aiming to use genome editing technology to modify the genes of specific biological species. We are also moving forward with the development of technology focused on how accurately we can modify genes, and are seeking to use the incredible technology of genome editing for “manufacturing” that will have positive effects on both people and the environment.
Hiroshima University has an abundance of scientists who have been involved in genome editing since the early days of the technologys introduction in Japan, and has amassed a wealth of know-how. In 2019, we launched the university startup PtBio Inc., which is equipped with functions for both the development of genome editing technology and the analysis of organisms’ genomic information (Figure 1). Having received commissions and investment from commercial companies, we provide genome editing technology optimized to the purpose of use, and are undertaking numerous projects and joint studies. We aim to provide the best consulting services and reliable technology, so that researchers in a variety of fields can adapt to genome editing technology and make swift progress in their research.
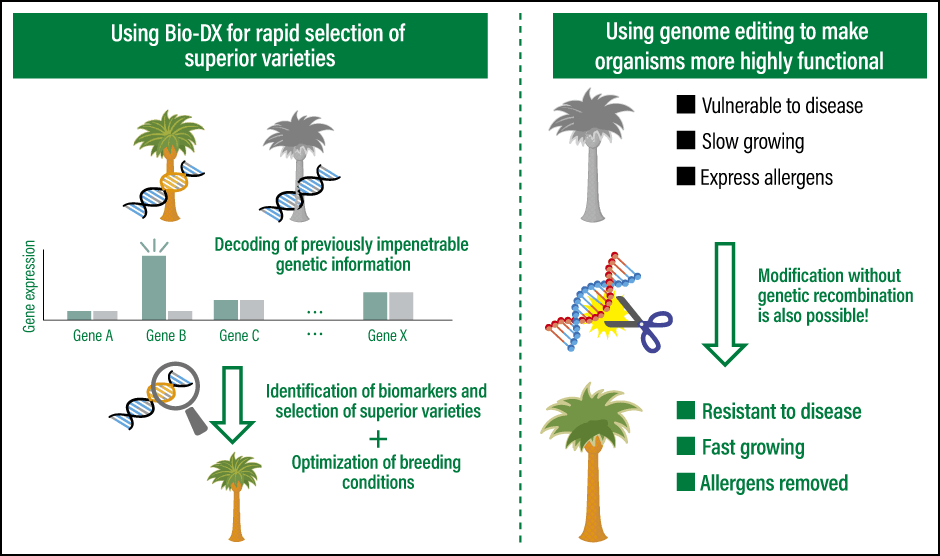
Figure 1. Solutions developed by Hiroshima University startup PtBioBio-digital transformation (Bio-DX) using proprietary AI or big data and the like is helping to reduce the time required to select superior varieties, which formerly took many months or years, and facilitating the use of cutting-edge genome editing technology, thereby enabling scientists to make organisms more highly functional. The aim of bio-DX is to solve social issues.
Thanks to genome editing technology, foods with increased added value are starting to be sold commercially, including tomatoes with a high accumulation of a functional compound, and sea bream with increased muscle mass. As such, we are aiming to bring to market a genome-edited food in whose development our research team has been involved in two or three years’ time. In a joint research project also involving a team led by Professor Hiroyuki Horiuchi from Hiroshima University’s Graduate School of Integrated Sciences for Life, along with a major food company, we have developed an hypoallergenic egg by using Platinum TALENs to modify a gene, thereby eliminating one of the main allergy-causing substances, or allergens (Figure 2).
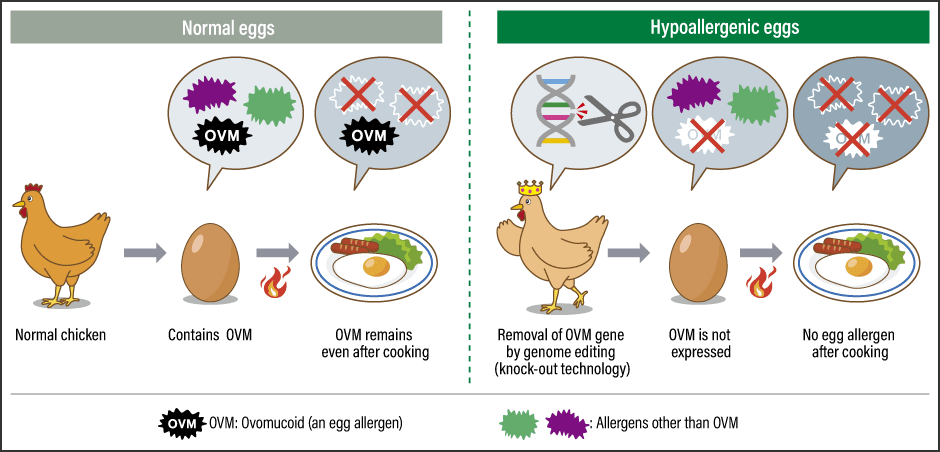
Figure 2. Comparison of normal eggs and hypoallergenic eggsOne of a few allergens in eggs, OVM does not lose its allergenicity even when cooked or processed using digestive enzymes. Using a genome editing tool, researchers produced chickens from which the OVM gene had been knocked out and confirmed that the eggs they laid did not contain OVM. Clinical research is progressing, with a view to bringing the eggs to market in two or three years’ time.
Egg allergy is the most common food allergy in Japan, with some research reports suggesting that around 10% of all babies are born with an egg allergy. The main allergens in eggs are ovomucoid (OVM), ovoalbumin, ovotransferrin, and lysozyme; apart from OVM, all the other allergens are vulnerable to heat, so their allergenicity is reduced if adequately cooked. However, as OVM is very stable even when exposed to heat or digestive enzymes, and is also water-soluble, it does not lose its allergenic properties even when cooked or processed using digestive enzymes.
Development of hypoallergenic eggs
Professor Horiuchi had been undertaking research into chickens for some time. Aware that many people suffer from an allergy to eggs, he had been addressing the challenge of producing eggs from which the allergens had been removed. Since 2013, he had been working on research and development focused on OVM-free eggs, using Platinum TALENs in a joint research project with a food company.
The first step involved producing chickens from which the OVM gene had been knocked out using Platinum TALENs. The team then confirmed that the eggs laid by the OVM-knockout chickens contained no OVM. They also checked that no concerning mutant proteins had been produced as a result of genome editing. No abnormalities were found in the external appearance of either the parent chickens or the eggs, nor did whole genome sequencing reveal any off-target effects.
As the hypoallergenic OVM-knockout eggs were thus shown to be safe, they have now entered the applied research phase, in which the team is conducting clinical research in collaboration with National Hospital Organization Sagamihara National Hospital to confirm their safety in patients with an egg allergy. Under the supervision of a specialist physician, the research team carried out an oral food challenge to test cooked and powdered hypoallergenic eggs in 17 patients with an egg allergy caused by OVM. All 17 individuals were placed under observation, but no allergic reaction was found. The research team plans to carry out this clinical research on a larger number of individuals by 2026, including conducting multiple tests in which the quantity of egg consumed is gradually increased.
The development of hypoallergenic eggs is being undertaken with funding from the Japan Science and Technology Agency (JST), having become one of the Center of Innovation for Bio-Digital Transformation (Bio-DX) projects selected as a full-scale co-creation field in the JST’s Program on Open Innovation Platforms for Industry-academia Co-creation (COI-NEXT) in 2022. Hiroshima University is the representative agency of the Center of Innovation for Bio-DX, and I serve as the center’s project leader.
Through the promotion of Bio-DX, the Center of Innovation for Bio-DX aims to realize a bioeconomy society that enables sustainable development without leaving anyone behind. Guided by this aspiration, it seeks to leverage both genome editing technology and digital technology for decoding and analyzing genetic information in order to maximize biological functions, and solve problems faced in such areas as food, health, and energy.
One joint research project we are undertaking in the medical field is the development of treatments for inherited retinal diseases in collaboration with a research team led by Dr. Masayo Takahashi, President and CEO of Vision Care Inc., which is a leader in research and development in the field of retinal regenerative medicine using induced pluripotent stem cells (iPS cells).
This group of diseases is caused by gene mutations, which cause progressive degeneration in the photoreceptor cells and retinal pigment epithelium, eventually resulting in the death of these tissues. The most common of these diseases is retinitis pigmentosa, which is one of the causes of acquired sight loss and is estimated to afflict approximately 1.5 million people worldwide. There is currently no cure for the condition, only supportive treatment aimed at delaying progression, in the form of drug therapy, including vitamin tablets and drugs to improve blood flow, and assistive devices such as light-shielding glasses.
Social demand for increasingly advanced genome editing technology
Hopes are high that genome editing technology will assist in delivering treatments for certain types of retinitis pigmentosa, and a joint research project involving our team has begun. We have developed an efficient method of designing highly specific ZFNs (ZF-ND1) (Figure 3) and achieved cleavage efficiency comparable to CRISPR-Cas9 in adult photoreceptor cells. In addition, as ZFNs have a lower molecular weight than CRISPR-Cas9 and TALENs, we believe that we can establish a gene delivery technique using adeno-associated viral vectors, so we are conducting further research in this area.
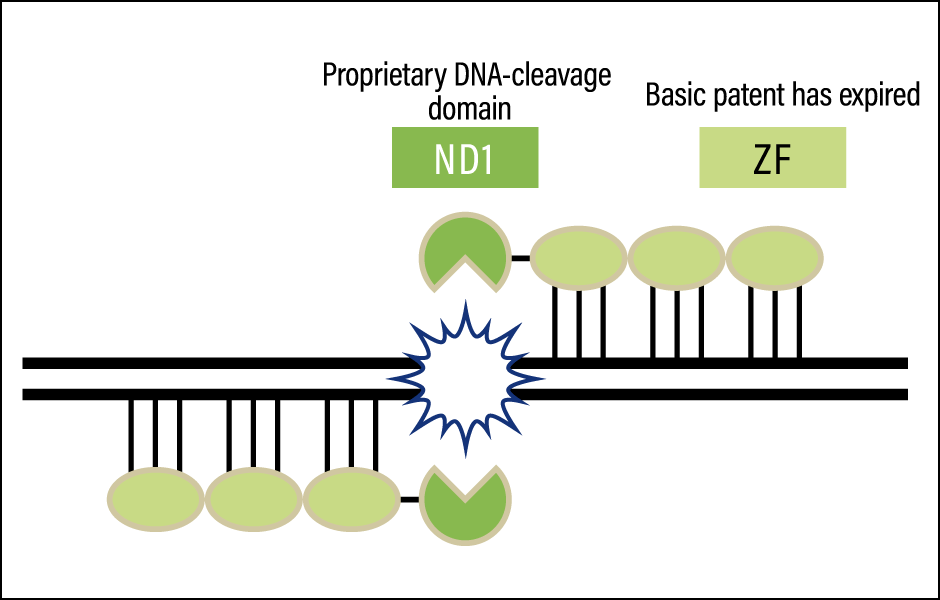
Figure 3. The genome editing tool developed at Hiroshima University: ZF-ND1The basic patent on the DNA-cleavage domain ZF in the first-generation ZFNs has expired, and can therefore be used without a license. Professor Yamamoto and his team have created ZF-ND1, a purely Japanese-produced genome editing tool that combines ZF with FirmCutND1, the proprietary DNA-cleavage domain that they developed. Industrial use of this tool is likely to accelerate, as it avoids the need to pay expensive license fees.
Thus, scientists in Japan and overseas are continuing to tackle a variety of challenges with the aid of genome editing technology. However, the deep-seated sense of antipathy toward foods produced by recombinant DNA techniques appears to be as strong as ever in Japan. The root cause of this stems from misunderstandings that arose in the absence of careful explanations concerning gene recombination, as well as a lack of adequate discussions involving a diverse array of stakeholders that also included sociologists and civic groups, rather than researchers and company representatives alone.
To start with, quite a few people are skeptical about how genome editing technology differs from gene recombination. There is a plant breeding technology that brings about natural spontaneous mutations at numerous sites in the genome by exposing plants to radiation. Scientists then focus on a mutation at a single effective site and undertake selective breeding based on that. As genome editing can bring about a mutation at a single target location, it would be fair to say that it is a safer technique, scientifically speaking. We researchers painstakingly strive to explain to people that we never allow organisms containing another organism’s DNA to reach the market, and that we only supply items whose safety has been thoroughly checked. However, I feel we need to make further efforts in this regard. If we properly communicate with the public and ensure they understand, I believe social acceptance of genome editing technology will develop and its industrial use will become more widespread.
At the same time, I think there is great significance in the fact that, as in the case of hypoallergenic eggs, genome editing technology can help children who suffer from egg allergies. My view is that social acceptance will grow as a result of creating the advances that people have been longing for that would not have been possible without genome editing technology. This has been my abiding belief ever since I first became involved in genome editing technology.
Genome editing technology as a tool for adding a high level of value by delivering faster growth and higher yields, is an advantage only for producers, and not for consumers. I believe customers will accept new technologies and be able to enjoy their benefits if we produce things that would not have been possible without genome editing technology. We are positioning hypoallergenic eggs as the first of these.
Right now, we are all astonished by the climate change causing major disasters on an unprecedented scale. In the climate that is developing, the agricultural crops that have conventionally been grown in Japan will not thrive. Selective breeding for resistance to high temperatures and dry conditions will be needed, and the use of genome editing technology will be a major tool in this.
The same applies to the environmental problems provoking climate change. One challenge where we hope to achieve success at the earliest possible opportunity is that of energy production using microorganisms based on genome editing technology, as a means of producing energy that does not rely on petroleum or other fossil resources.
Genome editing technology is now a tool in our armory. I am keen to make full use of this tool in taking on the challenge of resolving the social issues faced by humanity.