Enabling grease to be washed away efficiently, surfactants are an essential part of our daily lives. However, as almost all of them are produced from petroleum, their discharge in wastewater at one time became a major problem for society. While the widespread use of water treatment reduced serious contamination in Japan, petroleum-based surfactants continue to impose a burden on the environment. Research is underway into biosurfactant that is derived from microorganisms and easily degrades in the natural environment.
Special Feature 1 – Microorganisms and Sustainability Eco-friendly biosurfactant production
composition by Takakazu Kawasaki
Even if they are unfamiliar with the word “surfactant,” everybody knows what bubbles and foam are. You will have observed them when you have lathered up soap, blown soap bubbles, whisked up a salad dressing, or seen sea foam at the beach. Bubbles and foam are the phenomenon in which surfactants stick to the boundary between a gas and a liquid, forming a sphere.
In Japanese, the word awa refers to both bubbles and foam. Bubbles are formed when you blow air into a liquid, creating a gas particle, or air bubble, within the liquid. Foam, on the other hand, is the phenomenon in which these air bubbles reach the surface of the liquid without bursting and stick to the bubbles around them, forming a layer of froth in an endless stream.
Foam is important because we believe that both research into defoaming and research into the creation and use of foam will help to reduce the environmental burden, thereby contributing to the realization of a sustainable society. The use of surfactants produced by microorganisms could increase in the future. As surfactant substances are contained in liquids used in the manufacture of a variety of items, including pharmaceuticals, cosmetics, foods, fibers, paints, plastics, and paper, dealing with the foam generated in the manufacturing process is a very troublesome problem that affects product cost and quality alike.
Surfactants enable water and oil to mix
You may wonder why foam is so troublesome.
To explain the importance of foam disposal, let us first start by discussing the action of surfactants.
For example, no matter how much you agitate them, water and oil will eventually separate out over time. In short, the reason why water and oil do not mix is because of the big difference in their surface tension. In both water and oil, their respective molecules cluster together and pull at each other. As this cohesive force (surface tension) is much greater in water than in oil, they do not mix.
However, there are substances that make water mix with oil: surfactants. The “surf-” part of the word refers to the boundary surface where two different liquids —— such as water and oil —— meet, or between a gas and a liquid, as in the case of a bubble. Substances that act on this boundary surface to transform the properties of the liquid or liquids are called surfactants.

As surfactants gather on the surface of the bubble membrane, at the boundary between a gas and a liquid, they become concentrated into foam with adjoining bubbles.
When we do laundry, mud-soiled clothing will come out clean even if washed without soap. However, oil-based soiling from food or the sebum secreted by our skin, for instance, cannot be washed away using water alone. That is why we use laundry detergent, which is a surfactant.
The molecular structure of surfactants is depicted as a shape similar to a matchstick with a big, round head. The part equivalent to the stick is called the hydrophobic tail (lipophilic tail), while the part forming the round match head is called the hydrophilic head. As the names suggest, the hydrophobic tail mixes well with oil, while the hydrophilic head blends readily with water. One attribute of surfactants is their propensity for gathering at the interface (boundary) between oil and water. A surfactant molecule measures just one 500,000th of a millimeter.
When we do laundry, we place clothing soiled with sebum or oil in the washing machine, add water and detergent (principally composed of surfactants), and agitate them. The surfactant’s hydrophobic tails form spheres around small lumps of sebum and oil, and the hydrophilic heads form burr-like protrusions pointing outward. As the hydrophilic head has a high affinity for the water in the washing machine, the sebum and oil dissolves into the water in a way that they would not readily do otherwise, leaving the clothes clean.
A similar phenomenon can be seen with mayonnaise. Mayonnaise is made from vegetable oil, vinegar, egg yolks, spices, and other seasonings. Vinegar has similar properties to water. You cannot make mayonnaise simply by agitating vegetable oil and vinegar. But if you add egg yolks and then agitate the mixture, the lecithin (a phospholipid) in the egg yolk acts as a surfactant, surrounding the vegetable oil and forming a colloidal state with the vinegar that does not readily separate again.
Microorganisms that make surfactants
Returning to the subject of bubbles, as surfactants gather at the boundary between a gas and a liquid, they become concentrated into foam with adjoining bubbles. I wondered whether there might be some way to put this foam to good use.
Some microorganisms make surfactants. These microorganisms emit surfactants from their cells. A buildup of surfactants in liquid outside the cells causes clusters of bubbles to form on the liquid’s surface, due to the nature of surfactants. Along with many research institutions and companies, I am currently undertaking research into biosurfactants (surfactants made by microorganisms) (Figure 1). The development of biosurfactants and a cheap method for manufacturing these environmentally friendly detergents is a pressing issue. If biosurfactants can be manufactured cheaply, we will be able to reduce our use of surfactants reliant on petroleum-based ingredients that do not break down easily.
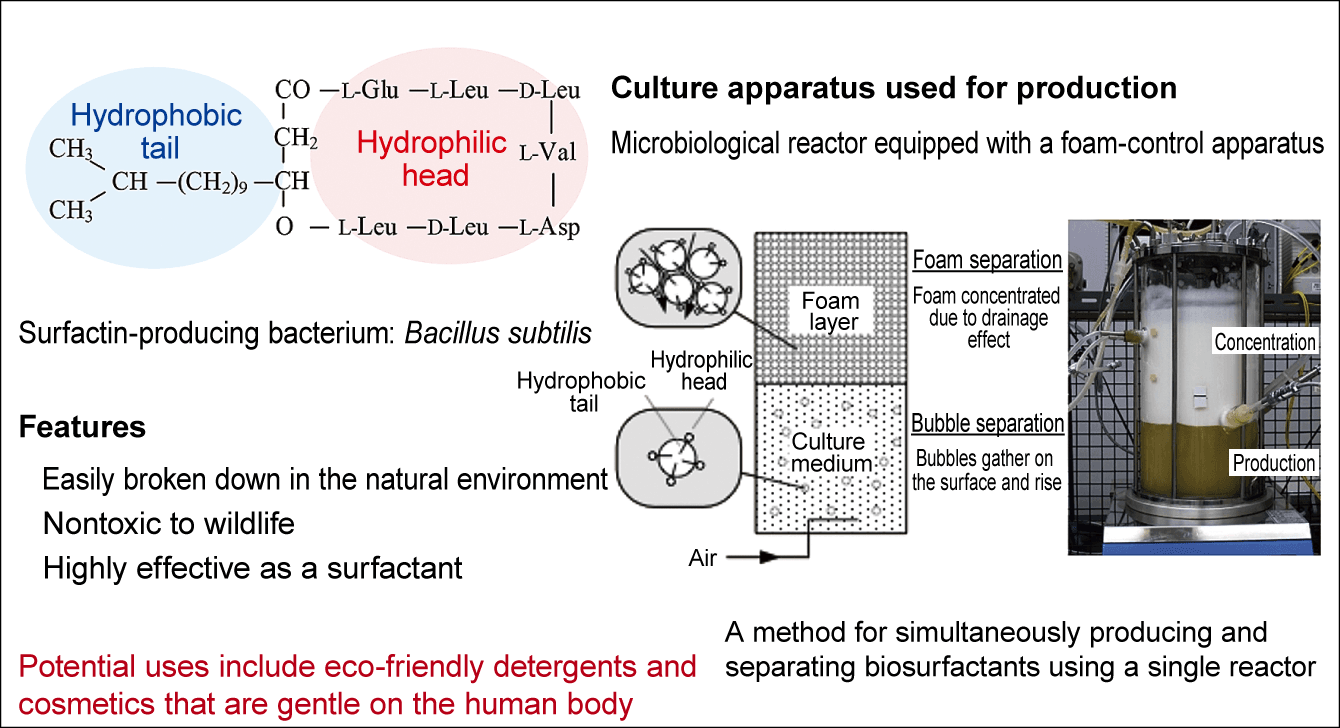
Figure 1. Biosurfactants (surfactants produced by microorganisms)When culturing microorganisms that produce biosurfactants, a layer of foam forms on top of the culture medium when air is introduced to the culture medium. As the biosurfactant concentrated in this foam is the product, it is necessary to control foaming to avoid overflow from the tank.
In my laboratory, we allow surfactant-producing microorganisms to proliferate in a tank of liquid, which results in the surfactant they produce collecting in foam. We then skim off the highly concentrated foam, from which we can extract the surfactant concentrate that is the target substance. Once the solution to be extracted reduces in volume as it becomes more concentrated, separation becomes more efficient.
To produce large quantities, we increase the capacity of the tank and the volume of liquid inside, and then culture the microorganisms. A great deal of foam is produced in the culture process, and it builds up into a bulky mass, we have to reduce the volume of liquid to ensure it does not spill out of the tank. Being unable to use the tank to its full capacity entails losses. These losses drive costs up. And if the foam overflows and spills out of the tank, we cannot produce the surfactant we need. Controlling foam production requires advanced technology.
Only the other day, I received an inquiry from a company about ways to minimize foam production, to which I responded online. Although they were not able to provide me with a lot of detail, because company secrets were involved, the information I did receive suggested to me that it would be tricky to curb the generation of foam.
Substances that reduce surface tension
My laboratory’s research into the production of biosurfactants began with the bacterial strain Bacillus subtilis natto. An undergraduate research project served as the catalyst for this. One of my students was researching what happens to the amylase (starch-degrading enzyme) produced by Bacillus subtilis natto, looking at such questions as whether or not it moves into the foam, and if it does, how much transfers into the foam. To increase the amount of amylase, the student bought nattō —— soybeans fermented with Bacillus subtilis natto —— and cultured the bacterium from it. While supervising this student’s research, I noticed that the surface tension of the Bacillus subtilis natto culture medium had declined drastically.
The surface tension of water is 72 mN/m and that of the culture medium before culturing the bacterium was a similar level. However, when we measured the culture medium after culturing the Bacillus subtilis natto, we found that the surface tension had dropped to around 30 mN/m–less than half that of water.
Why did this happen? Having formulated the hypothesis that some kind of substance that reduced surface tension was being produced, we investigated the substances in the culture medium. The results showed that the substance lowering the surface tension was a surfactant called surfactin (a cyclic peptide produced by Bacillus subtilis natto).
The microorganism Bacillus subtilis natto is a type of hay bacillus, and the surfactin produced by it was discovered in the latter half of the 1960s. Major chemical manufacturers were already researching ways of using and manufacturing surfactin. Surfactin has a highly surface-active effect even in minute quantities and it penetrates the skin easily. Today, taking advantage of these properties, it is used in cosmetic cleansers and lotions.
Incidentally, the surfactant produced by Bacillus subtilis natto is not a component in nattō’s distinctive slimy texture. That slime is actually a substance called polyglutamic acid.
Various other companies have consulted me, saying that they want to try making biosurfactants. I think this is because there is a strong consumer preference for products made from environmentally friendly, naturally derived ingredients, particularly in European countries. Even in Japan, a growing number of consumers choose products out of consideration for the environment, even if they are a little more expensive than others. I believe this tendency is part of a global trend.
One of my research themes is how to make surfactants efficiently. In fact, I got started in this field by researching how to destroy foam when I was an undergraduate. I obtained my Ph.D. for research into an apparatus that prevented foam from spilling out of the tank, using both mechanical and physical methods. Today, I continue to study defoaming technology for mechanically removing problematic foam generated by industrial production processes.
Soap bubbles and the saponin bubbles that form when boiling soybeans are only generated in small quantities, so they can be eliminated by lowering the temperature or adding water. In contrast, if trying to get rid of foam generated in a large industrial plant, it is necessary to use either a mechanical method involving a foam-breaking apparatus or a chemical method involving an antifoaming agent. The former is my field of specialism.
Foaming and defoaming technology
A common type of foam-breaking apparatus uses rotating blades to break up the foam. Other devices use such approaches as a rotating disk or spraying a mist to reduce the foam. I employed the knowledge of defoaming technologies that I had gained through my research when I was involved in the development of an energy-efficient wet painting booth (a painting booth that uses foam) (Figures 2 and 3).
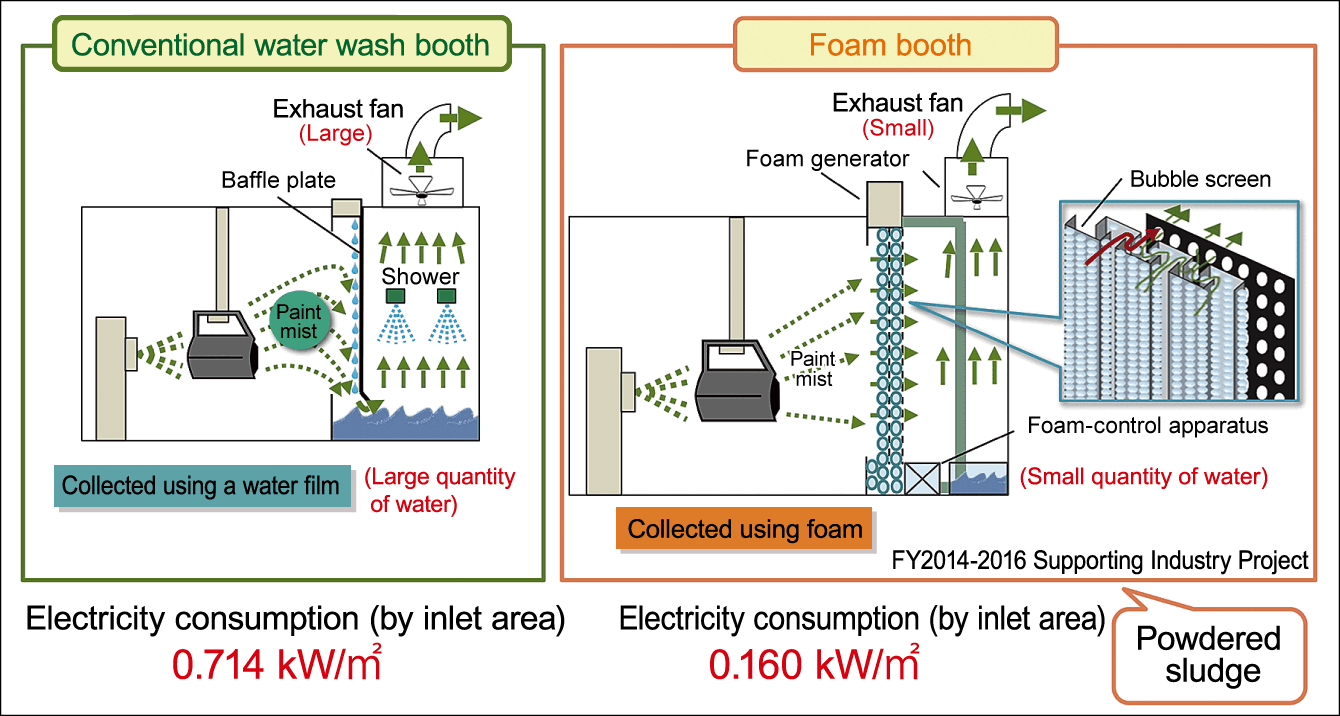
Figure 2. Energy-efficient wet painting boothThe energy-efficient wet painting booth in whose development Professor Takesono was involved. The paint mist generated in the painting process is collected by spraying it into foam that has been generated, which is then expelled from the booth. The foam booth consumed less electricity and generated less noise when running than conventional water wash booths.
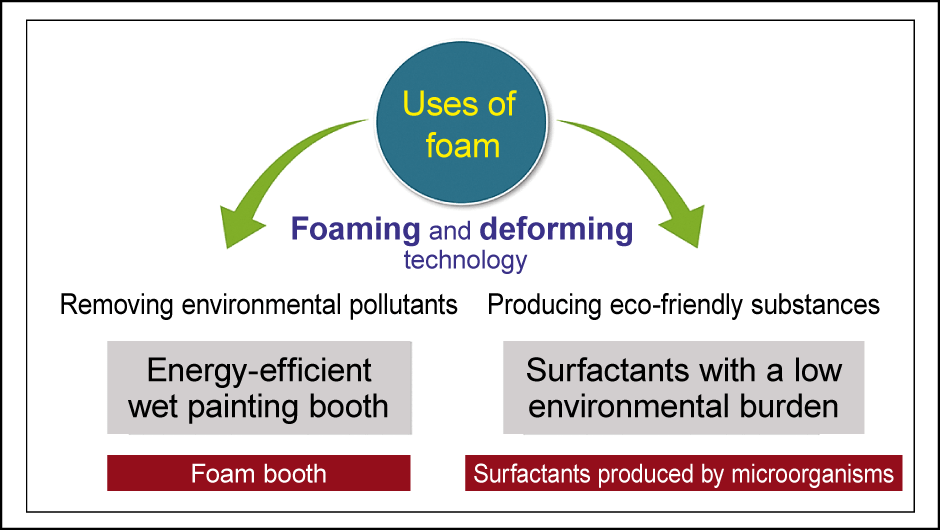
Figure 3. Using foam to reduce environmental burdensBoth research into defoaming (foam booth) and research into making and using foam (efficient production of biosurfactants) contribute to reducing the burden on the environment.
One example of the use of the chemical method that might be familiar to you is the use of an antifoaming agent in the production of tofu. As mentioned above, the foam is covered with a surfactant of the same type to preserve the balance of the surface tension. If a surfactant of a different kind from the one in the foam gets near it, the second surfactant may penetrate the surfactant in the foam. If this occurs, the balance of the foam’s surface tension will be destroyed and the bubbles will readily burst if subject to just a slight vibration or shock. Surfactants that act in this way are used as antifoaming agents.
The cell membrane has a similar structure to a bubble. The cell membrane has a phospholipid bilayer with the hydrophobic tails facing each other. This structure is similar to surfactants having a hydrophobic and a hydrophilic side. (Figure 4). While these membranes are robust, the external membranes of viruses (known as the envelope) are destroyed by medical alcohol with a concentration of at least 70%. This is why the use of alcohol disinfection is recommended to combat COVID-19.
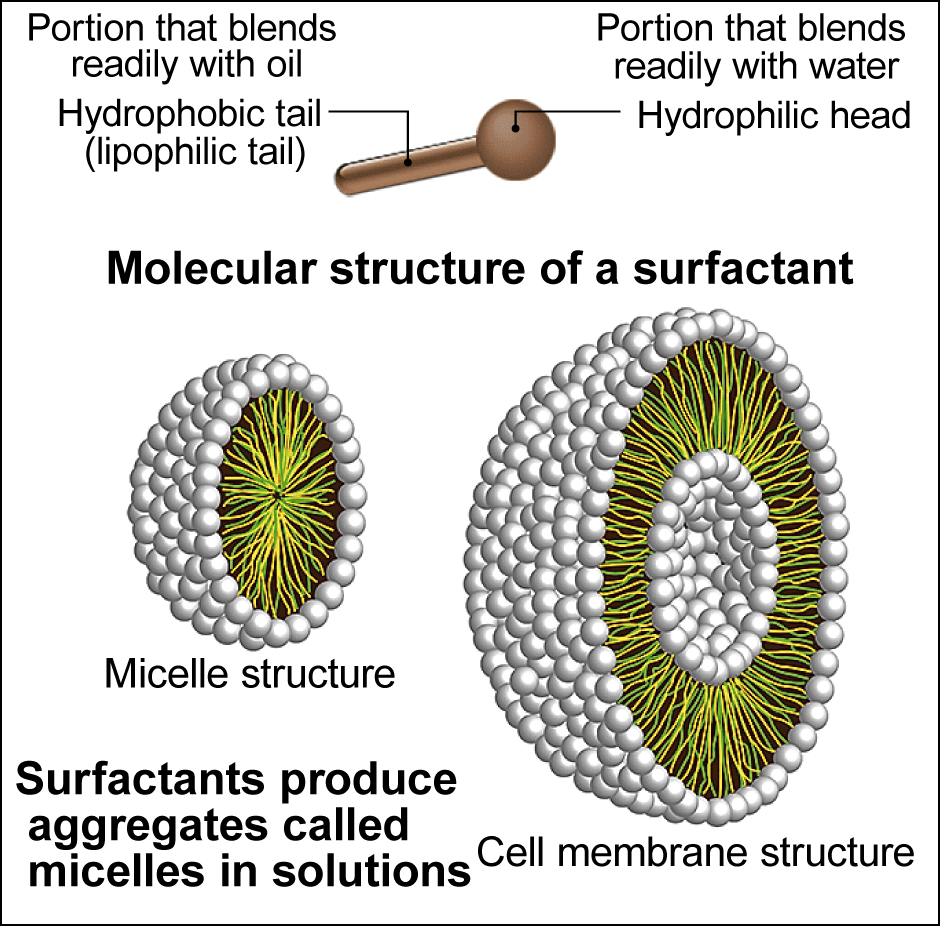
Figure 4. Surfactants and micelle structureIn micelles (left), the hydrophobic tails gathers facing inward, while the hydrophilic heads are in contact with water, whereas cell membranes (right), have two layers of phospholipids.
Children love bubbles. They are especially overjoyed when you blow soap bubbles big enough for them to stand inside. When my children were little, I used to add a small quantity of sugar to ordinary dishwashing liquid to make soap bubbles. Adding sugar results in bigger bubbles that are more resistant to breaking. The sugar clings to the water molecules around it and does not easily release them. Adding sugar to bubble soap curbs the evaporation of moisture from the bubble membrane, so the bubble is more resistant to breaking.
I plan to continue researching both technology for producing foam and technology for reducing it. Bubbles and foam really are fascinating.