Peptides often feature in the ingredient lists of foods and cosmetics, but what actually are they? Aside from peptides of these kinds, our own bodies contain peptides produced when proteins synthesized from amino acids are broken down. This diverse array of peptides has a variety of hormonal effects demonstrating powerful physiological activity. As they are molecules that already exist within the human body, they are less toxic than chemically synthesized drugs and have therefore been attracting attention in the drug discovery field for some time.
Special Feature 1 – The World of Peptides Spotlighting a diverse array of functions beyond hormonal effects
composition by Rie Iizuka
illustration by Koji Kominato
Peptides are molecules that bind together two or more alpha-amino acids (hereinafter “amino acids”). These amino acids are basically composed of a single carbon (C) atom in the middle, which is bound to an amino group (NH2) and a carboxyl group (COOH). Figure 1 shows two amino acids called alanine and glycine. When the amino group of one of these amino acids and the carboxyl group of the other become dehydrated (lose their H and OH, which become H2O) and bond, they form a peptide. The peptide coupled these two amino acids together still has NH2 at one end, which can bond with the COOH of another amino acid by means of dehydration. This is how amino acids connect with each other to form high molecular weight peptides. The rule of thumb commonly holds that a chain of up to 99 amino acids is a peptide, while one consisting of 100 or more is a protein, but the reality is that the boundary between proteins and peptides is quite blurred, with some chains of 200 amino acids still being considered to be peptides.
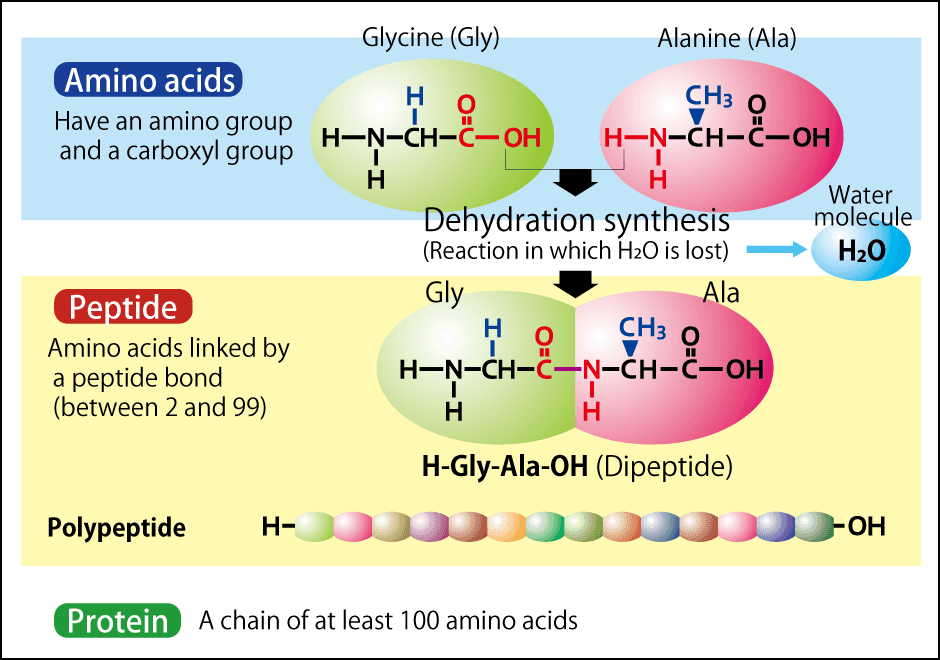
Figure 1. Amino acids and peptidesA single peptide bond results when dehydration synthesis occurs between two amino acids, leading to the loss of H2O. A chain of two amino acids is called a dipeptide, one consisting of three is called a tripeptide, and one made up of four or more is called a polypeptide.
Peptides and proteins are categorized by function
Peptides and proteins are categorized not only by the number of amino acids of which they are composed, but also by their function. For example, cells require a framework to create and maintain their shape, and the huge peptides providing that framework are classified as proteins. Peptides with enzyme activity are also classified as proteins. This is because, in order to function as enzymes, they have to both be at least a certain size and have a certain shape to secure sufficient space to act like a factory incorporating specific substrates and producing the relevant chemical reaction. However, the protease produced by human immunodeficiency virus (HIV) is a very small enzyme consisting of 99 amino acids. Its size therefore means that it should be classified as a peptide, but it is actually classified as a protein, because it is an enzyme (its actual enzyme function is expressed as a dimer).
On the other hand, among the peptides in the body is, for example, growth hormone, which consists of some 180 amino acids. By number alone, it falls into the realm of proteins, but because it functions as an endocrine hormone, it is classified as a peptide.
So, how are peptides made in the body? Rather than being directly synthesized through the bonding of amino acids, the first step in their formation is the creation of large proteins (precursors) from the amino acids. These are then cut at specific locations by special enzymes, and these fragments become peptides (Figure 2). There is an enormous number of types of peptides. The proteins in the human body are composed of 20 different amino acids, so 20 × 20 = 400 different dipeptides can be created by connecting just two amino acids. In reality, there are considerably more peptides, because a lot more amino acids are joined together. However, as the peptides required by our body are regulated by genes, the number is not unlimited.
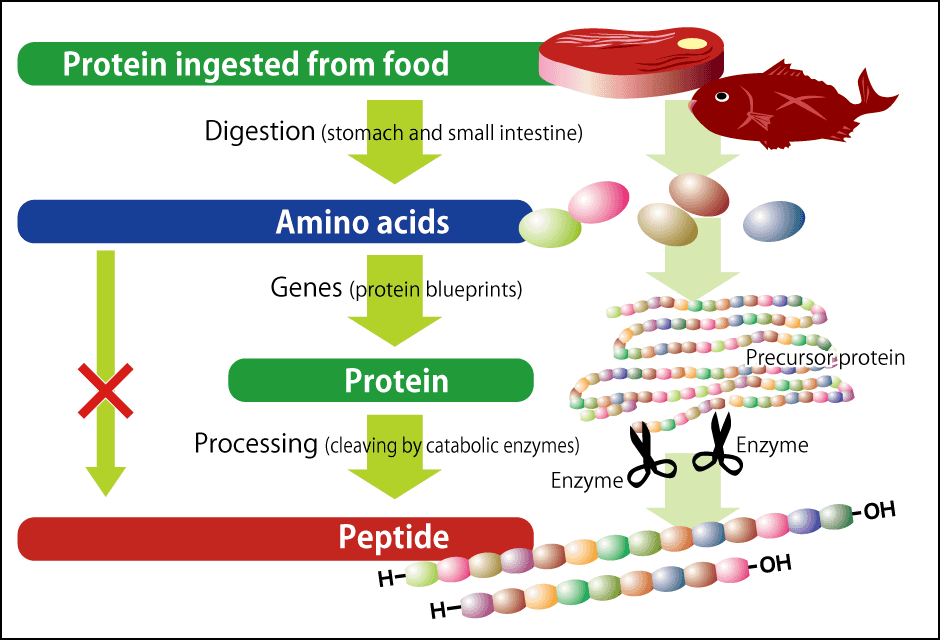
Figure 2. Protein and peptide synthesis in the bodyWhen we eat food, it is broken down into amino acids in the gastrointestinal tract. These are used to synthesize proteins or make proteins to produce peptides.
Peptide hormones are secreted by various organs in the body, but those in the brain are mainly secreted by the hypothalamus and pituitary gland. First, the hypothalamus secretes several hypothalamic peptide hormones. When these reach the pituitary gland, the pituitary gland releases several different pituitary peptide hormones throughout the body.
For example, when the hypothalamus secretes the peptide called growth hormone-releasing hormone, which consists of 44 amino acids, this stimulates the anterior pituitary to release growth hormone into the body, causing our bones to lengthen and making us taller.
Drugs that tap into the hormonal effects of peptides
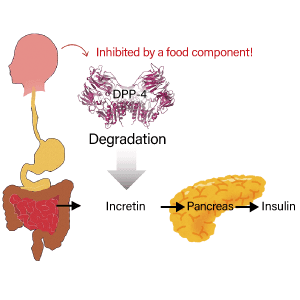
Many drugs that tap into these hormonal effects of peptides have been available for some time. For example, the insulin secreted by the pancreas is a peptide hormone consisting of 51 amino acids. Cells throughout the body have gates that let in the blood sugar required to produce energy. Insulin serves as the key that opens these gates. Consequently, insulin is an essential drug to treat diabetes, which causes high blood sugar. About 20 years ago, attention began to focus on a peptide hormone called GLP-1, which promotes the secretion of insulin from the pancreas. Drugs based on this peptide hormone are now used to treat patients with type 2 diabetes.
Recently, drugs designed to prolong the effects of GLP-1 in the body and peptide-based antidiabetic drugs for oral administration have also begun to appear. One factor hindering peptide-based drug discovery has been the fact that drugs incorporating peptide hormones are digested, making them unsuitable for oral administration. However, by slightly altering the chemical structure of GLP-1 and binding it to specific molecules, these drugs prevent GLP-1 from being broken down in blood, enabling it to accumulate in the body and thereby prolonging its effects.
Rather than using peptides themselves, some drugs use a technique called peptidomimetics, which involves mimicking the chemical structure of the peptide to boost its effectiveness as a drug. This drug discovery technique was employed in the development of drugs to combat HIV/AIDS.
Once HIV infects human immune cells, the first thing it does is to make two giant viral proteins in our cells. Then, a proteolytic enzyme from the virus itself (HIV protease) cleaves these giant proteins at specific points to produce 13 viral proteins, causing a single infected immune cell to make numerous HIV particles. The mechanism for spreading the infection through the body works through the discharge of these particles from the cell. To put it the other way around, if the enzyme can be stopped from breaking down the giant proteins at the initial stage, it will not be possible to make HIV particles in an infected cell and the virus will be unable to reproduce. Accordingly, to obstruct the effects of HIV protease, medicinal chemists designed the theoretical chemical structures for drugs based on small peptides that mimic the amino acid sequence near the points at which the giant proteins are cleaved and then developed inhibitors that would cause the protease to lose its function as a hydrolytic enzyme. These inhibitors cling fast to HIV protease and cannot be dislodged. HIV protease inhibitors are a leading example of the successful use of peptidomimetics in drug discovery (Figure 3).
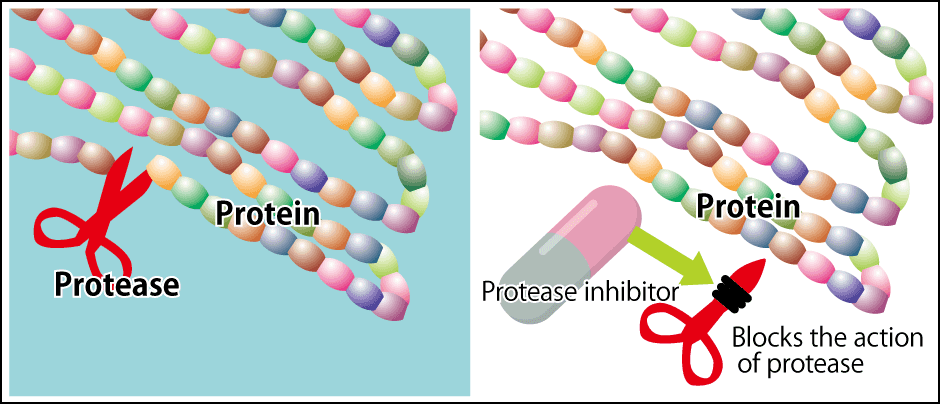
Figure 3. Peptidomimetics and the HIV protease inhibitor mechanismPrevents the proliferation of HIV by blocking protease when HIV cleaves the proteins.
There are several conditions that make a chemical structure ideal for pharmaceuticals. First, smaller molecules are more easily absorbed, ensuring they can exist stably in the body and therefore rendering them more effective. Peptide molecules, however, are comparatively large. In addition, oral administration is less invasive for the patient than injections, but peptides are generally unsuitable for oral administration, because they are metabolized by digestive enzymes. Similarly, as various proteolytic enzymes are found in our blood and organs, it is not easy to imbue peptides with a reasonable degree of durability. The aforementioned peptide hormone GLP-1 can only be maintained in the body for around five minutes and the fact that its effects are strong enough to reach even the peripheral areas of the body nonetheless is a testament to its powerful efficacy. Although difficult to handle, peptides are characterized by a very high level of specificity and selectivity when it comes to the site of action of a drug. Moreover, as peptides are molecules found in our bodies, they are less toxic than ordinary chemically synthesized drugs, but they do have the drawback that they are very expensive to manufacture.
Usage trends are changing
In drug discovery, peptides mainly serve as hormonal components, enzyme inhibitors, or protein-protein interaction (PPI) inhibitors. PPI refers to the exchange of information that occurs in cells when proteins bind to or separate from each other. When this occurs, peptides can be used as an inhibitor by inserting themselves between the proteins to block their interaction. As proteins are large substances, the binding interface involved in PPI is also large, so drugs with a low molecular weight of less than 500 were unable to cover such a broad surface, making it difficult to inhibit binding. However, medium-sized molecules with a molecular weight of around 500 – 2000 can block the process, so peptides began to attract attention as PPI inhibitors. In my view, trends in the use of peptides in drug discovery are shifting from drugs based on the hormonal effects of peptides to the creation of PPI inhibitors.
Peptides are also used in food. Aspartame is probably the only chemically synthesized peptide used in food. Consisting of two amino acids–aspartic acid and phenylalanine–bound together, this peptide is said to be between 100–200 times sweeter than sugar. It is approved as a chemically synthesized food additive. Naturally derived peptides are used in items sold as food for specified health uses.
In addition, many peptides are used as bases for cosmetics; you have probably often heard the name “collagen peptides.”
As touched upon at the beginning, peptides are created in the body when proteins made from amino acids are cleaved. In the case of collagen, after the protein has been created, most of one of its amino acid components, proline, is oxidized by an enzyme, introducing an OH structure and turning it into hydroxyproline. Collagen is a giant molecule; tendons such as the Achilles tendon could basically be described as bundles of collagen. However, collagen does not dissolve in water. Accordingly, foods containing collagen contain fragments of peptides resulting from collagen being cleaved by various enzymes. When ingested, these are basically broken down into amino acids in our gastrointestinal tract. It has recently been suggested that dipeptides (derived from two amino acids) and tripeptides (derived from three) are easier to absorb from the gut, so when digestive function is impaired, such foods are effective in promoting the intake of amino acids.
However, collagen peptides are not used directly by the body as collagen after being eaten. In other words, whether you eat food said to contain collagen or whether you eat tofu, amino acids are basically supplied in the same way in both cases. Although people say collagen is good for the skin, there is still no established scientific evidence to show that eating a lot of collagen is good for the skin.
In addition to those with hormonal effects, many peptides with antibacterial effects, such as defensins, are known to exist. Broadly speaking, antibacterial peptides have a positive charge and demonstrate their antibacterial effect by acting on the cell membranes of the target microorganisms, which have a negative charge. Some biological species have 100 or more antibacterial peptides effective against a wide range of microorganisms. Another interesting point is the fact that many species, from humans to microorganisms, have antibacterial peptides.
The diverse array of peptides produced by microorganisms
Cyclosporin is a peptide that suppresses human immunity. Consisting of 11 amino acids, it is a very powerful immunosuppressant. It deactivates the immune system by suppressing production of interleukin-2, a cytokine that promotes the activation of T cells, which have an immunological effect. A peptide obtained from microorganisms, cyclosporin contains special amino acids not among the 20 types of amino acids that make up the proteins coded by human genes. As microorganisms build peptides using special enzymes, they sometimes contain amino acids never encountered in the human body. Research into such peptides has been progressing rapidly of late, driving intense growth in the field of biosynthetic chemistry, which focuses on creating peptide molecules with various novel structures by using the Escherichia coli bacteria to artificially alter the chemical structure of peptides produced by microorganisms. I believe these findings will be fed back into drugs in due course.
Peptides are fundamental substances that we encounter in all aspects of our daily lives.