The peptides we ingest as food are composed of various compounds, which have a diverse array of functions relating to the nutrition, taste, and bioregulation of foods. They have a higher absorption rate than proteins and amino acids and are known to be the most efficient means of building muscle, for example. They are also involved in the most important element of food —— taste —— and studies currently underway aim to equip peptides with bioregulatory functions governing such matters as blood pressure and glucose metabolism.
Special Feature 1 – The World of Peptides Nutrition, taste, and bioregulation —— Peptides’ diverse functions under the spotlight
composition by Toshiko Mogi
Chains of between 2 and 99 amino acids are called peptides. Those consisting of 100 or more are generally called proteins (Figure 1).
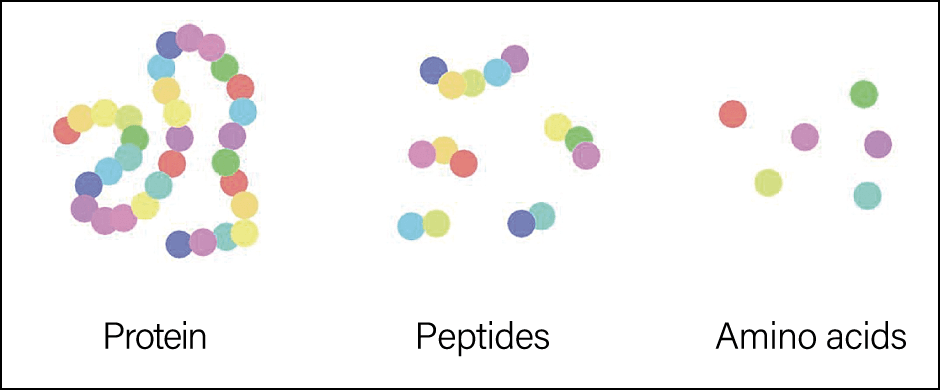
Figure 1. Peptide structureA diverse array of peptide functions are constructed from the 20 different amino acids in our bodies, determined according to their composition and the order in which they are linked to each other.
When we eat protein, it is broken down into peptides and ultimately amino acids. However, even before they are eaten and broken down, our foods contain various peptides. For example, when we eat fermented foods such as cheese, yogurt, nattō (fermented soybeans) and miso, we are actually eating proteins that have been broken down into peptides by microorganisms.
While meat is rich in protein, it also contains peptides. Accordingly, peptides enter our system from the moment we put a piece of meat in our mouth, and once it has been digested, our body makes more peptides from the protein in the meat.
Thus, we consume peptides in the course of our everyday lives in familiar foods, rather than in a special form.
Peptides are absorbed most efficiently
Food has three functions: nutritional functions, sensory functions, and bioregulatory functions. Examples of foods in which nutritional functions are of primary importance include sports drinks, nutrition drinks, and the enteral nutrients used in medical settings. Foods whose main function is sensory include treats such as sweets and alcohol. And those with bioregulatory functions are so called health foods, such as foods for specified health uses (FOSHU) and foods with function claims.
Consisting of 20 different types of amino acid in various configurations, peptides are not a single molecule (compound). As they contain various compounds mixed together, their functions are also diverse. Consequently, they too are closely related to the three functions of food.
When we eat protein, it is broken down in our stomach and then broken down further in the small intestine before being absorbed. There are two patterns via which this takes place: absorption after being broken down into amino acids, and absorption part-way through the process, when still at the peptide stage. Peptides are absorbed more quickly than amino acids.
For instance, whether we eat soy protein as protein, peptides, or completely broken down into amino acids —— that is to say, where the composition is the same and only the size of the molecules differs in the three cases —— we know that peptides have the best absorption efficiency.
Due to this feature related to their nutritional functions, peptide-based ingredients are widely used in such items as sports foods and enteral nutrients, where the outstanding absorbability of a source of amino acids is a key requirement.
In the mechanism via which peptides are absorbed into the body as a source of nutrition, molecules called peptide transporters catch peptides and carry them throughout the body. The main one found in humans is peptide transporter 1 (PEPT1) in the small intestine.
However, the term “peptide” covers a huge variety of combinations of amino acids. For example, there are as many as 8,400 dipeptides and tripeptides, which respectively consist of just two or three of the 20 different types of amino acid in the human body. How does the body select which ones to absorb?
The tendency of a transporter to recognize and transport a wide variety of substance groups is called substrate multispecificity. The detailed mechanism involved in the substrate multispecificity of peptide transporters was hitherto unclear. In 2013, our research team shed light on part of the mechanism for the first time worldwide.
Having developed our own technique, which was simpler than conventional methods, we conducted experiments to investigate which peptides were more easily absorbed. Our results revealed that only peptides consisting of two or three amino acids were absorbed by a transporter. This transporter is called a proton-dependent oligopeptide transporter (POT). We discovered that this transporter recognizes the type of peptide and then swiftly and efficiently absorbs peptides containing the essential amino acids required by the body. We also determined that not only humans, but also all other living things, from microorganisms to higher animals and plants, have this system.
Peptides mainly associated with taste
Our finding that smaller peptides containing essential amino acids are more efficiently absorbed as a source of nutrition is contributing to the enhancement of enteral nutrients and sports foods. Particular progress has been made in the development of the latter. While it is important to eat protein as a source of nutrition for building muscle, the most efficient means of doing so is to consume it in the form of peptides. Nowadays, there are many protein supplements on the market containing whey peptides, which are made from milk. At the same time, I and many other researchers are still working to shed light on the substrate multispecificity of peptide transporters.
Sensory functions relate to how tasty food is. For example, we cook meat to make it taste good. However, protein basically has no taste. When cows and pigs are slaughtered, rigor mortis causes the meat to harden, but after a while, it softens and its umami increases. This is because the protein is broken down, increasing the quantity of amino acids and peptides. In other words, it is not that the protein itself tastes good, but rather that the increase in amino acids and peptides resulting from the protein being broken down makes the meat tasty. When meat is cooked, the reaction between amino acids and sugars give rise to the Maillard reaction and Strecker degradation, generating flavor components that whet the appetite. We experience this complex interaction of taste, flavor and smell as the tastiness of meat.
Thus, the tastiness of food is a sensory experience derived from such factors as taste and smell. Peptides are principally associated with flavor. Determined according to the composition and sequence of amino acids, there are many different types of peptides, so we do not completely know which peptides have which tastes. However, they are basically said to contribute to the depth of flavor of foods —— that is to say, fullness, or thickness, or richness of flavor. However, when food manufacturers develop peptide ingredients, they usually taste terrible. They are bitter. Why is that?
The bitterness of peptide ingredients is an issue
Humans can differentiate five basic tastes, which are shown in the table along with their roles. Of the five basic tastes, the one to which we are most sensitive is bitterness. This is because when a bitter-tasting substance enters the body, it could be poisonous. If, for example, a protein is broken down into one sweetness peptide, one umami peptide, and one bitterness peptide that are evenly distributed, we will sense the bitterness more strongly than the sweetness or umami, because we are highly sensitive to bitterness.
| Sweetness | A desirable taste that also acts as a sign that a source of energy is entering the body |
| Umami | The taste of the materials of which the body is composed |
| Sourness | The taste enabling us to identify rotting food |
| Saltiness | The signal for detecting minerals |
| Bitterness | The signal for detecting poisons |
Table. The five basic tastesThe human sense of taste can be divided in physiological terms into five categories: sweetness, umami, sourness, saltiness, and bitterness. Each plays an important role for the body.
Humans do not eat things that are bitter. Solving the problem of peptide ingredients’ bitterness has been a key challenge. One example can be seen in the intensely sweet flavor added to enteral nutrients. The term “enteral nutrition” means the administration of essential nutrients via the gastrointestinal tract – orally or by tube feeding. While the peptide molecules in these are intentionally kept small —— just two or three amino acids —— as their primary purpose is to increase absorption efficiency, smaller peptides have more intense flavors, so they have a stronger bitter flavor. An intensely sweet flavor is added to such nutrients to drown out the bitterness.
When developing foods containing peptide ingredients, manufacturers have devised various ways of achieving a milder flavor, including altering the type of proteins used to make the peptides and changing the way in which they are degraded, but a fundamental solution remains a long way off. It was against this background that we discovered a bitterness-suppressing peptide through our research.
Bitterness occurs because the bitterness peptide binds to molecules found in the taste receptors (taste buds) in our mouths. In the course of a study we are conducting into how humans sense taste and smell, we discovered a peptide that suppresses the action of the bitterness receptors that we need to perceive bitterness. Conversely, we also conducted research into using peptides to eliminate bitter components. Catechins, which are most commonly known as the healthy component of green tea, are also a component of bitterness. Catechins bind to the bitterness receptors, causing the sensation of bitterness. However, when the relevant peptide is bound to catechins, they become unable to act on the bitterness receptors. The mechanism works by binding the peptide to the catechins before they reach the receptors, ensuring that we do not experience bitterness.
The 2014 National Health and Nutrition Survey revealed that about 73% of respondents stated that tastiness (flavor) was the aspect to which they attached importance when choosing foods. Flavor —— including not only making tasty foods, but also making unpleasant-tasting foods taste better —— is vital to maintaining health through diet. Research into the relationship between peptides and flavor is being conducted to investigate which peptides activate or suppress which taste receptors (Figure 2).
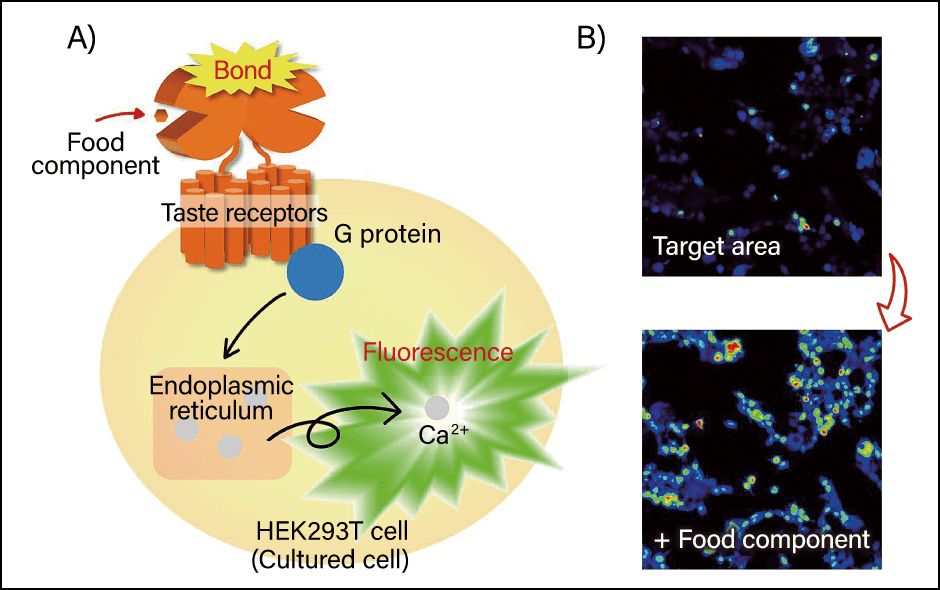
Figure 2. Outline of an experiment to investigate the bonding of food components to taste receptorsA) Schematic diagram of an experiment to express taste receptors in cultured cells and quantitatively analyze changes in intracellular Ca2+ concentration due to a food component. B) The cell lights up when the receptors are activated by the food component, causing various signals to be transmitted within the cell.
A prime example of the bioregulatory functions of peptides is angiotensin-converting enzyme (ACE) inhibition. When a peptide called angiotensinogen is released from the liver and broken down by renin, it becomes angiotensin I. Angiotensin I is broken down by ACE into angiotensin II, whose effects on the body raise blood pressure. Consequently, inhibiting the action of ACE prevents blood pressure from rising. The ACE inhibitors used in treating high blood pressure have quite a similar chemical structure to peptides. FOSHU and foods with function claims have been developed for people concerned about their blood pressure using foods containing peptides with a similar chemical structure to angiotensin-converting enzyme inhibitors.
Aiming for effects meeting the FOSHU standard
As there are many different types of peptides, we cannot definitively state that a particular peptide has a specific effect. However, if asked, “are there peptides with this specific effect?,” the answer would, generally speaking, be “yes.” It has already been reported that there are peptides that increase hair growth, peptides that improve cognitive function, peptides that suppress fat absorption, and peptides that inhibit sugar absorption. But do they actually work?
Even if a peptide has one such effect as a substance, it is broken down in the body after being eaten, becoming oligopeptides and amino acids that are then absorbed, so they often cannot get as far as the target in the body. Accordingly, even if a peptide demonstrates an effect in the test tube, if I were asked “Would it be effective if I actually ate it?,” I would have to say that the barriers to its being effective are quite high.
FOSHU and foods with function claims containing ACE inhibitory peptides have been confirmed as acting on the target through clinical studies and other rigorous investigations of their effects. While various research efforts are underway in other areas, there has not yet been any success in reaching the standard required to be designated as FOSHU.
Amid this situation, we are working on the development of DPP-4 inhibitory peptides. When we eat, our gastrointestinal tract releases molecules called incretins. These are a type of peptide hormones. When incretins are released, they act on the pancreas, causing it to secrete insulin, with the result that sugar is absorbed into the body from blood vessels (Figure 3).
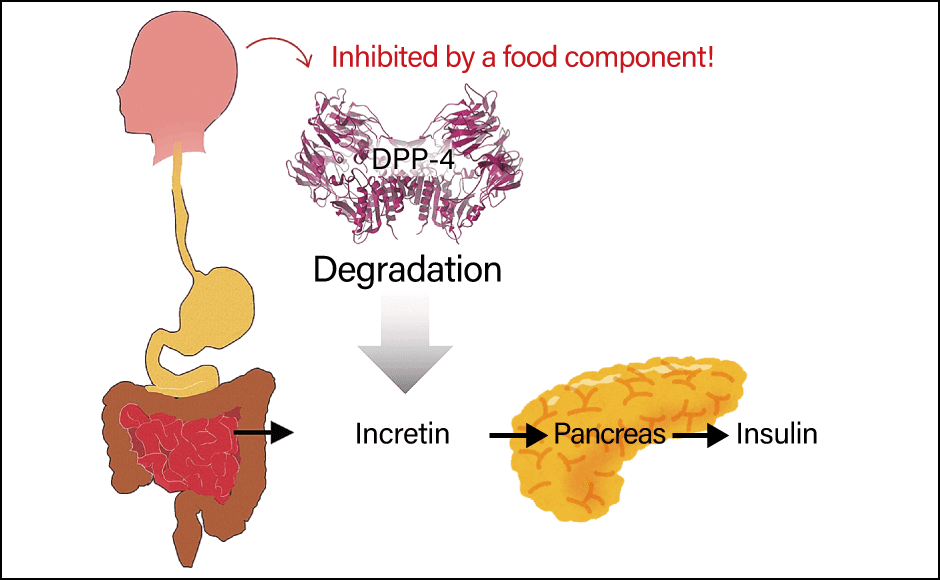
Figure 3. DPP-4 and its inhibitionIncretin is the key to the mechanism whereby insulin is secreted and lowers blood glucose. Hopes are high for a functional peptide capable of preventing type 2 diabetes by inhibiting the incretin-decomposing effect of DPP-4.
However, an enzyme called dipeptidyl peptidase 4 (DPP-4) breaks down incretin in the space of just five minutes. This reduces the duration of insulin secretion, ultimately reducing the quantity secreted and making it hard to control blood glucose. Based on this mechanism, drugs called DPP-4 inhibitors were created to treat type 2 diabetes. As people with diabetes do not secrete enough insulin, the purpose of inhibiting DPP-4 is to promote insulin secretion and thereby alleviate their diabetes. Accordingly, food components with a DPP-4-inhibiting effect were explored by a number of researchers, including our team. We started by causing microorganisms to produce DPP-4 and then searched various food components for those that would suppress the enzyme’s effects. As a result, we discovered that one type of peptide had a powerful suppressant effect on DPP-4.
As ACE inhibitory peptides have already been approved as a FOSHU, we know that peptides consumed orally enter the blood vessels and exert an inhibiting effect on enzymes in blood vessels. As DPP-4 is an enzyme found in the blood vessels, this logically suggests that a similar effect would apply. It has already been proven to be possible in animal research. However, as clinical studies are required to satisfy the criteria for approval as a FOSHU, it could take a while before this particular peptide becomes available to the public.
As we have seen above, the peptides that serve as a source of nutrition with high absorption efficiency have a diverse array of functions, and it is hoped that they will become useful as tasty peptide ingredients to promote health.