Sugar is an important nutrient that serves as a source of energy, and is crucial to maintaining physiological functions. As such parts of the body as the brain, nerves, and red blood cells are unable to use any sugars other than the monosaccharide glucose, it would be fair to say that sugar truly is a fundamental molecule with a direct connection to life itself. Its flavor and properties are essential in cooking, and its sweetness broadens the pleasure of eating. As such, sugar is an important presence in food, too. It also has a diverse array of other functions, including acting as a preservative, as it inhibits lipid oxidation, starch retrogradation, and protein denaturation. Understanding sugar properly and consuming an appropriate amount is the secret to staying healthy.
Special Feature 1 – Focus on Sugars Sugar is essential to life: A proper understanding will keep us healthy
composition by Rie Iizuka
illustration by Koji Kominato
Sugar is a source of energy. Consisting of one atom of carbon (C), which could be described as the origin of life itself, with one water molecule (H2O) attached, this substance is represented by the chemical formula Cm(H2O)n (Figure 1).
- *1 Excluding sugar alcohols.
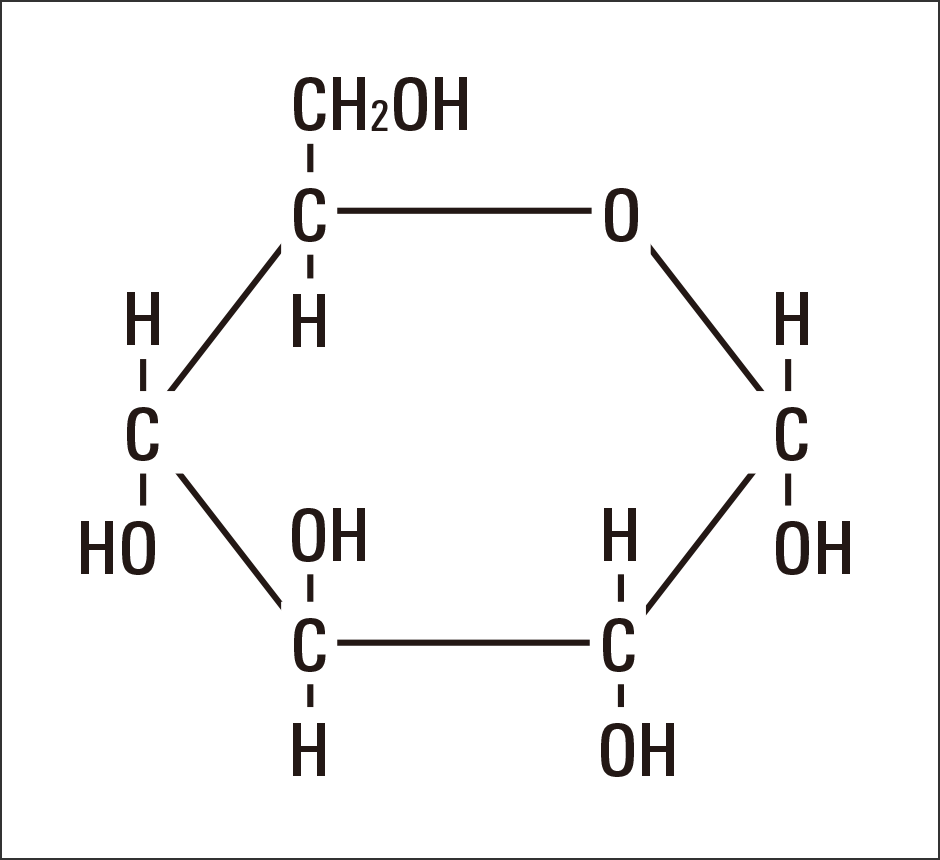
Figure 1. The chemical structure of glucoseGlucose, the most basic monosaccharide, has the structure Cm(H2O)n, being composed of one carbon (C) atom joined to one water molecule (H2O). It is a primitive and highly efficient element structure in which carbon is emitted as energy and H2O is ultimately emitted as water.
Playing a key role in activities that support life
Sugar ingested as a food is digested and broken down into simple sugars (monosaccharides) such as glucose and fructose. These are absorbed in the intestines and carried throughout the body in the blood, providing energy. Energy can be produced by nutrients other than sugar, namely protein and fat. However, from the perspective of the organisms’ metabolism, carbohydrates are the most efficient means of obtaining energy.
In addition, red blood cells do not have mitochondria, which can convert fat into energy, while the brain has the blood-brain barrier —— a barrier function that rigorously guards against toxic substances. Consequently, a key feature of these organs is that sugar (glucose) is the only substance they can use as an energy source.
Sugar not only serves as a source of energy, but also plays a key role in activities that support life as a structural component in cells. Cell membranes are composed of glycans consisting of connected monosaccharides, and glycoproteins and glycolipids, in which a monosaccharide is linked to a protein and a lipid, respectively. Sugar is also involved in the synthesis of amino acids, lipids, and nucleic acid, making it absolutely essential to the body.
Sugars are classified into a number of categories: saccharides (monosaccharides, disaccharides, and sugar alcohols), oligosaccharides, and polysaccharides (Table).
| Category | Subclass | Component substances | Digestibility | Classification in Dietary Reference Intakes for Japanese | |
| Saccharides | Monosaccharides Disaccharides Sugar alcohols |
Glucose, galactose, fructose, sucrose, lactose, maltose, sorbitol, mannitol | Easy | Carbohydrates | Sugars |
|---|---|---|---|---|---|
| Oligosaccharides | Maltooligosaccharides | Maltodextrin | |||
| Other oligosaccharides | Sucrose, lactose, trehalose, maltose (disaccharides), raffinose, panose, maltotriose, melezitose, gentianose (trisaccharides), stachyose (tetrasaccharide) | ||||
| Polysaccharides | Starch Non-starch polysaccharides |
Amylose, amylopectin, etc. Cellulose, hemicellulose, pectin, etc. |
Difficult | Dietary fiber | |
| Category | Subclass | Component substances | Digestibility | Classification in Dietary Reference Intakes for Japanese | |
| Saccharides | Monosaccharides Disaccharides Sugar alcohols |
Glucose, galactose, fructose, sucrose, lactose, maltose, sorbitol, mannitol | Easy | Carbohydrates | Sugars |
|---|---|---|---|---|---|
| Oligosaccharides | Maltooligosaccharides | Maltodextrin | |||
| Other oligosaccharides | Sucrose, lactose, trehalose, maltose (disaccharides), raffinose, panose, maltotriose, melezitose, gentianose (trisaccharides), stachyose (tetrasaccharide) | ||||
| Polysaccharides | Starch Non-starch polysaccharides |
Amylose, amylopectin, etc. Cellulose, hemicellulose, pectin, etc. |
Difficult | Dietary fiber | |
Also known as non-digestible carbohydrates.
Nutritionally speaking, they are referred to as carbohydrates, which are composed of sugar and dietary fiber (Figure 2).
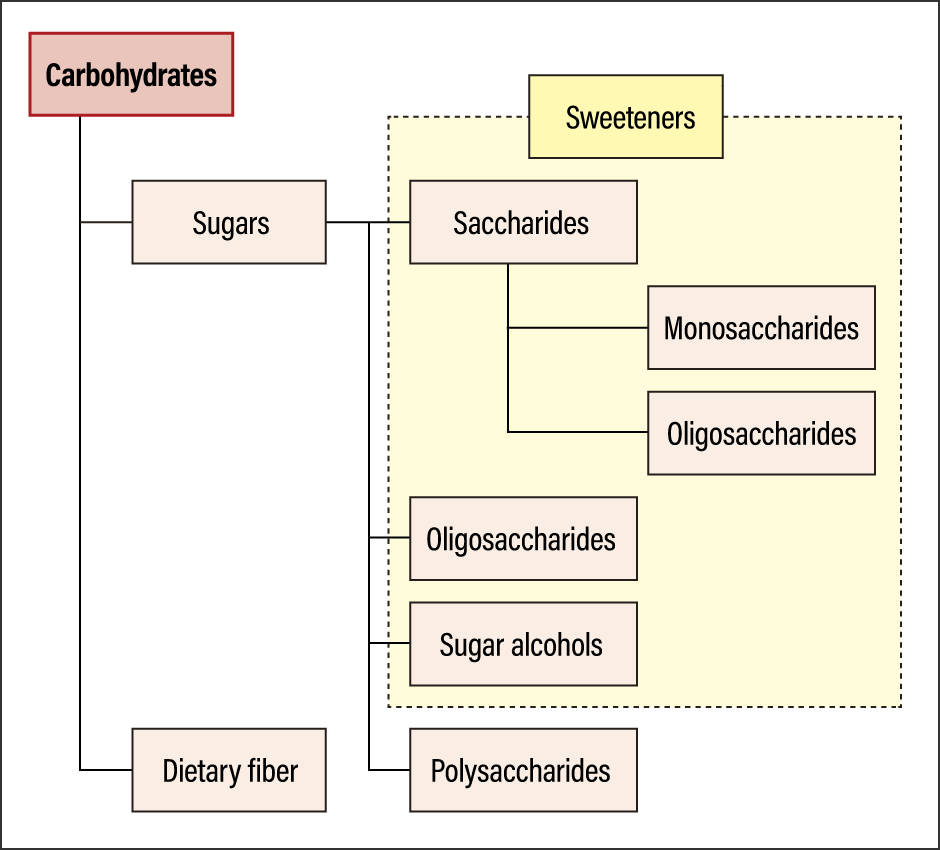
Figure 2. Classification of carbohydratesDietary fiber was formerly included in the carbohydrates category, but progress in analysis technology enables us to distinguish dietary fibers from other sugars. It was initially thought to be calorie-free, as it cannot be digested by humans, but the concept is evolving as a result of new discoveries, such as the fact that dietary fiber is broken down in the colon by the action of intestinal bacteria, converting it into energy.
The Standard Tables of Food Composition in Japan 2020 (8th Revised Edition) contain a revision relating to carbohydrates. In the 7th Revised Edition, which preceded the 2020 edition, the quantity of carbohydrates was calculated using the subtraction method; the remainder after deducting the total quantities of food components such as water, protein, fat, and ash. The 8th Revised Edition contains not only figures for available carbohydrates, but also figures for the carbohydrate components starch, monosaccharides, and disaccharides, which can be digested and absorbed in the human small intestine. The figures exclude dietary fiber, which is broken down by the normal microbiota resident in the human colon and used as a source of energy.
Oligosaccharides have a different mechanism of action in the body
The reason why nutritional science today classes both sugar and dietary fiber as carbohydrates is that the technology required to extract dietary fiber from carbohydrates did not exist at the time when the concept of carbohydrates was formed. At the same time, it was thought that carbohydrates contained a substance that promoted some kind of biological reaction. As analytical methods developed, scientists realized that, while sugar should basically be water-soluble, there was a component that did not dissolve in water. Further analysis resulted in the formation of the concept of dietary fiber.
Dietary fiber was initially regarded as containing virtually no calories. However, it was later confirmed that some level of energy is generated when dietary fiber is broken down in the colon by intestinal bacteria. Moreover, following analysis of low and high molecular weight water-soluble dietary fiber in food, the figures for dietary fiber carried in the Standard Tables of Food Composition in Japan (8th Revised Edition) have increased.
Sugar is classified into saccharides, oligosaccharides, sugar alcohols, and polysaccharides; apart from polysaccharides, all sugars are considered sweeteners. While oligosaccharides and sugar alcohols are classified as sugars in scientific terms, they are functional sugars, whose mechanisms of action in the body differ from those involved in energy generation.
Oligosaccharides is the collective term for molecules consisting of between two and 10 linked monosaccharides. While they are sweet, they are not readily broken down by the digestive enzymes in the small intestine, unlike other sugars, so they are sometimes classified as dietary fiber.
Sugar alcohols include naturally derived substances found in fruit and vegetables, and artificial substances synthesized based on the chemical structures and properties of naturally occurring sugar alcohols. For example, there are two kinds of xylitol: the substance derived from birch and beech trees, and the substance manufactured by means of chemical synthesis.
The only sugars that cannot be used as sweeteners are polysaccharides. The starch found in rice and bread falls into this category.
In recent years, foods and drinks touting their zero-sugar or zero-carb status have become popular in supermarkets and convenience stores. It is hard to distinguish these terms from each other, but the difference becomes clearer if we refer to Figure 2. The term “zero-sugar” means the item contains no sugar but does contain a sugar alcohol or similar as a sweetener. Zero-carb means the item also does not contain any polysaccharides. The Food Labeling Act stipulates that the content in 100 g of a food (or 100 ml of a beverage) must be less than 0.5 g.
If used skillfully, these products are handy for people who want to control their energy intake, but people should avoid choosing zero-sugar products in an emergency, such as when they have low blood glucose. Consuming a zero-sugar product when your blood glucose level has fallen temporarily due to having skipped a meal is dangerous, because the quantity of water in the body will increase, causing the blood glucose concentration to fall further. You should be fully aware that you will not recover from a low blood glucose situation unless you consume something like a soft drink containing natural fruit juice, or an item containing fructose or liquid glucose.
The preservative function of the sugar in wedding cake
The functions of sugars are utilized in cooking in various forms.
While adding sweetness is their best-known use, they also have a wide range of other functions, including maintaining the shape of a dish, adding texture, flavor or color, retaining moisture, inhibiting lipid oxidation, starch retrogradation, protein denaturation, and providing antiseptic effects.
Down through the ages, these functions of sugar have been used in a variety of culinary situations.
For example, wedding cakes are thought to have originated in mid-18th century Britain. The cakes used to celebrate marriages in modern-day Japan tend to be covered in cream, but traditional British wedding cakes are covered in icing with a high sugar content. Wedding cakes are often very big, consisting of as many as three tiers, and there is a historical reason attached to this. The custom was that the bottom tier was served to the guests at the wedding reception on the day, while pieces from the second tier were sent to those who could not attend the reception, and the third tier was eaten on a special occasion, such as when the couple’s first child was born or on their wedding anniversary. The quantity of sugar in each tier was varied, to adjust its preservative function.
Let us take a more everyday foodstuff: jam. Acid, sugar, and the water-soluble dietary fiber called pectin, which softens when heated, are used to preserve jam. Jam-making developed not only because of the sweet flavor of the result, but also from knowledge about how to preserve one’s produce in the longer term. Today, most jams tend to be runny, which is because advances in modern storage technology mean that the preservative function is no longer required.
The sweetness of sugars also varies quite widely, depending on the type and form of the sugar. Sucrose (table sugar) is hydrophilic, but although ordinary white sugar and rock sugar, for example, are both made from sucrose, rock sugar does not readily dissolve in water, so if you put it in your mouth, its sweetness is harder to sense, even though both types of sugar have the same level of sweetness. Similarly, brown sugar tastes sweeter than rock sugar, which is because the former has a high moisture content, making it easier for your tongue to sense the sweetness. The hydrophilia and solubility of sugars affect how sweet they taste.
Hydrophilia is also employed in another of sugar’s functions: viscosity, or glossiness. Boiling sugar and brushing it onto cookies, or onto meat or fish in a teriyaki sauce helps to enhance the appearance of the food, making it look tastier by producing a glazed look.
Sugars change in diverse ways, depending on the temperature range in which they are heated and how the moisture escapes. You might have seen spun sugar —— fine threads of candy —— used to decorate cakes. When sugar is heated to at least 140°C and dissolved, specialist tools and techniques can be employed to pull it into fine threads in order to produce delicate three-dimensional decorations.
Heating it further creates caramel. Caramel is sugar that has been browned to create a bitter flavor. If sugar is continually heated and browned, it can be used to impart color to sauces or provide umami when a small quantity is added to soups. If browned further until it creates a more intense caramel, it becomes a bitter seasoning that adds depth of flavor, rather than a sweet taste. Thus, in cooking, sugars have the power to substantially expand the horizons of food.
Now that we have established an understanding of the properties of sugar as a substance, let us look at its effects in the body.
Some sugars are synthesized in the human body as structural components in cells, while most are nutrients essential for the homeostasis of the body, providing the energy required to maintain body temperature, sustain the biological activity of organs and tissues via the beating of the heart, and keep the pH of the blood at around 7.4.
As touched upon earlier, the degree of reliance on glucose as a source of energy varies from one tissue to another, with the brain, nerve tissue, and red blood cells, among others, unable to use anything other than glucose. In terms of the daily glucose requirements of these tissues, the brain requires about 100 g, while nerve tissue and the blood together need around 50 g.
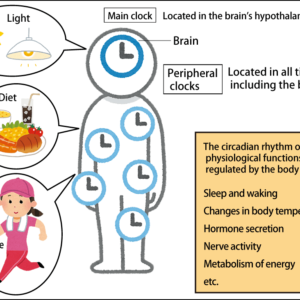
While consuming sugar to excess causes it to be stored as fat, the liver is the only organ in which sugar can be stored as glycogen. The body goes through a repeated cycle in which, when glucose is needed, the liver converts the glycogen and releases it into the bloodstream. However, the liver can only store a limited quantity of glycogen —— only about enough for one day. Consequently, constant consumption of sugar —— which provides the brain and the like with energy —— is required. We do not know for sure how frequently it should be consumed: three times a day, five times, or even just twice, as in 17th-century Japan. Nevertheless, consuming a uniform amount of sugar in at least two meals a day is regarded as a healthy level of consumption that does not burden the liver.
Appropriate intake if you are healthy and have a BMI of 18.5-25.0
Over the years, there have been many different theories about sugar intake. There is also plentiful research into dietary restriction for the purpose of weight loss, but eating habits and the local diet mean that Westerners are limited in their ability to restrict fat intake, while protein is necessary to replace muscle and blood components, so it is not possible for them to lose more than a certain amount of weight. Consequently, restricting sugar ends up being tabled for discussion.
However, comparing dietary research papers, I found that, while diets differed and subjects likely had received guidance on the recommended nutrient intake, such as protein, fat, sugar, and the like —— the nutrient ratio settled down within a certain range for all participants over time. 45-55% of the ratio consisted of carbohydrates, while the daily consumption of sugar was weighing in at 150-200 g. What can we conclude from this?
Ultimately, I regard this as likely to be the quantity desired by the body. Even when we talk about restricting the consumption of carbohydrates, which are a source of energy, there are limits to this.
Which then takes us back to the fundamental question of how much we should consume, but what is important first of all is to check whether our current energy intake is appropriate. I believe that what you eat at present is appropriate if your BMI is between 18.5 and 25.0 —— deemed to be a normal weight under the judgment criteria set by the Japan Society for the Study of Obesity —— and if your physique does not change a great deal and you have no health problems.
- *4 BMI: Body mass index. This index is used internationally as an indicator of obesity levels. It is calculated as an individual’s weight in kilograms divided by the square of their height in meters.
We can make a rough calculation of our energy intake from the amount we eat, so if carbohydrates make up 50-60% of our meals, they are within the appropriate range, broadly speaking.
For the sake of convenience, if we say that the amount of energy we require is 1000 kcal, deriving 50-60% of that from carbohydrates puts the amount of energy from carbohydrates at 500 to 600 kcal. Under the Atwater system of factors, there are 4 kcal in 1 g of carbohydrates, so the minimum daily intake of carbohydrates is 125 g.
- *5 Atwater system: A system of factors used to estimate the energy values (calories) of food.
Now that we have some rough figures, we can see that the traditional Japanese meal structure that emerged around 1700, based on rice, side dishes, and soup, makes it possible to eat a good balance of nutrients. In addition, in most Japanese families, each person around the dining table has their own specific rice bowl, I believe. As a result, we can consume more or less the same amount of carbohydrates every day. We can also adjust our intake, putting less rice in our usual bowl at dinner if we have eaten a large lunch. When it comes to consistent, optimal nutrient consumption, traditional Japanese eating habits could serve as guideline; both in terms of nutrient balance and quantity.
There are a number of studies about sugar intake methods and health, but we have been able to see from our own research that Japan’s traditional style of eating, based on one soup and three dishes, is closely related to Japan’s healthy life expectancy, including dementia prevention. This diet has been refined over centuries and I believe that it is also an excellent example of Japanese food culture from the perspective of regulating the balance of sugar in our diet.