The “dosage and administration” indicated on general non-prescription drugs does not distinguish between men and women. However, some prescription drugs recommend different doses, etc. by sex. Although studies on arrhythmia side effects have always been conducted in drug development, the severity of side effects differs by sex as sex hormones are associated with arrhythmia. Because of this, drug treatment and safety research based on gender-specific medicine is making progress.
Special Feature 1 – Diversity in Sex Drug safety research based on gender-specific medicine
composition by Takakazu Kawasaki
Gender-specific medicine is science which tries to elucidate physiological and clinical differences in diseases between men and women.
Having studied the cardiac toxicity of drugs, I intend to advocate gender-specific pharmacology to clarify whether there are sex differences in drug safety, and if there are, to warn about them.
Sex differences in drugs and causes of death
What are sex differences in drugs?
When we look at the “dosage and administration” indicated on general non-prescription drugs, there are no differences in the usage of drugs between men and women as their bodily functions are mostly the same.
On the other hand, sex differences become an issue with some prescription drugs. For example, in the case of the sleep inducer “zolpidem (brand name: Myslee),” the recommended dose for women has been reduced to half the dose for men since 2013. A sleep inducer should be effective when falling asleep, but it should not remain until the next morning. If zolpidem remains in the body, it is extremely dangerous to drive a car. Zolpidem is said to take a longer time to be eliminated in women than in men, so the dose for women is set at half the amount for men.
We can also see sex differences in causes of death.
In advanced countries including Japan, the top three causes of death are mainly lifestyle-related diseases. However, the top three slightly change when we look at the causes of death among Japanese individuals by sex (Table 1).
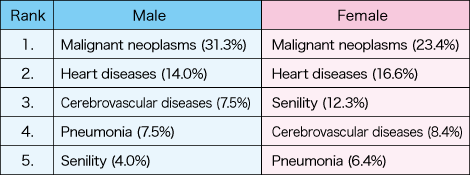
Table 1. Causes of death in JapanWhile the top cause of death in Japan is malignant neoplasms and the second is heart diseases in both male and female, the subsequently ranking causes differ by sex. The effects and safety of some drugs also differ by sex.
(Ministry of Health, Labour and Welfare, “Summary of Vital Statistics Report 2018”)
In women, senility ranks higher than in men at the third place. However, given that the average period of long-term care for women is longer than for men, sex differences in circulatory system diseases, such as a cerebrovascular disease, are also considered to be important factors in causes of death.
In the past, heart infarction and heart diseases were thought to be related to metabolic syndrome and more prevalent among men. In order to reduce the risk of these diseases, the United States implemented a cholesterol-lowering policy, which indeed pushed down men’s mortality rate dramatically. Nevertheless, this policy had no effect on women, so the mortality rate from these diseases became higher for women than for men in the United States by the beginning of the 1980s.
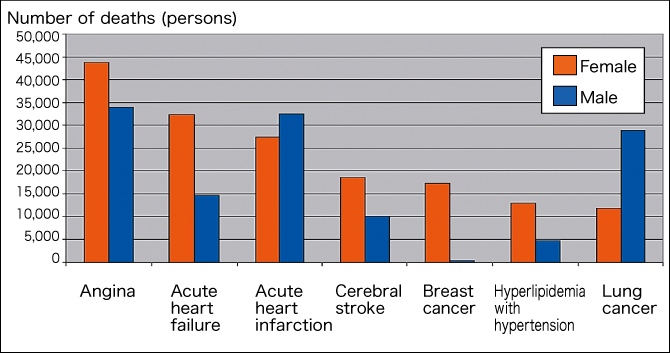
Figure 1. Sex differences in various diseasesCirculatory system diseases are related to aging, and show marked sex differences. This prompted the emergence of gender-specific medicine in the 1980s.
(Statistisches Bundesamt 2007; B Babitsch, GiM Charité Univ.)
Figure 1 shows German data indicating that the prevalence of angina, acute heart failure, cerebral stroke, and hyperlipidemia with hypertension is higher in women than in men. Circulatory system diseases are related to aging, and show marked sex differences. Gender-specific medicine emerged in Germany in the 1980s, and came to be studied in various other countries including the United States and Japan.
Ginseng having a myocardial protective effect
Around that time, I was studying the relationship between ion channels expressed in the heart and arrhythmia. Ion channels are membrane proteins embedded in the cell membrane of the heart muscle, which selectively filter ions inside and outside of cells, and transmit information on heart movement using electric signals.
Subunits of ion channels are made up composed of approximately 200 to 2,000 amino acids, and mutations of genes encoding them lead to increased susceptibility to arrhythmia. The focus of my research included how gene mutations change the function of ion channels and on which specific sites drugs for suppressing arrhythmia worked.
One day, a Chinese student from the Inner Mongolia Autonomous Region of China said, “ginseng has strong myocardial protective effects.” So, we decided to study the components of ginseng, and found ion channels acting in a peculiar manner by one of the components.
Until then, ion channels were considered to function in a specific way as a result of drug binding to the ion channels or due to their phosphorylation by a protein kinase (an enzyme that catalyzes the transfer of a phosphate group to a protein in a cell). However, we found that a ginseng component causes sex hormone receptors in cells of the heart to release nitric oxide (NO), and that this NO works on ion channels.
Nevertheless, we do not know the mechanism as to why heart muscle cells have sex hormone receptors.
Study on sex differences in drug treatment rapidly gained momentum due to a report issued by the U.S. General Accounting Office (currently Government Accountability Office) in 2001. According to a list of drugs withdrawn from the U.S. market (1997-2000) provided in the report, eight out of the ten drugs withdrawn from the market due to adverse events (side effects) showed significant sex differences. What is more, three of them posed a risk of inducing Torsades de Pointes (potentially fatal irregular heartbeat), which is a severe side effect that could lead to sudden death. In addition, five of the eight drugs had side effects on the circulatory system, including Torsades de Pointes and valvular heart disease (Figure 2).
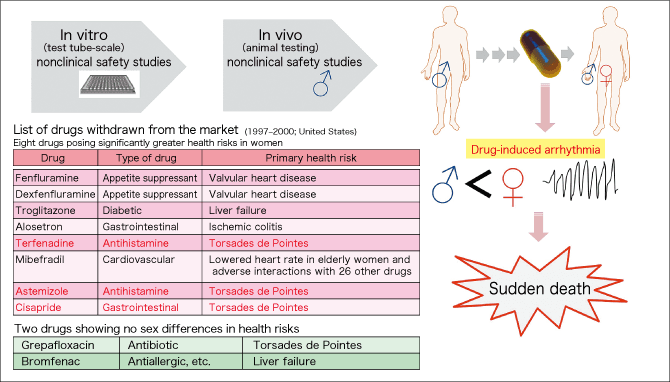
Figure 2. Sudden cardiac death/drug-induced arrhythmiaA report issued by the U.S. General Accounting Office in 2001 indicated that eight out of the ten drugs withdrawn from the market during 1997-2000 due to adverse events (side effects) showed significantly greater health risks in women. Five of the eight drugs had side effects on the circulatory system in women, and three of them posed a risk of inducing Torsades de Pointes, which could lead to sudden death.
Why were these severe side effects on women not identified in prior clinical trials? Drug safety studies are conducted first by in vitro (test tube-scale) molecular-level testing, and then by animal testing. The animals used in the testing are mainly rodents and dogs. However, as clinical trials in humans had previously caused a social issue in the case of thalidomide (causing fetal malformations), safety studies were basically conducted on adult men first, and then in a small number of patients, after which the drugs entered the market. Safety studies had not been conducted on adult women.
Electrocardiogram showing sex differences
I mentioned earlier that side effects of drugs withdrawn from the U.S. market were mainly those associated with the circulatory system, such as heart failure. Thus, let us take a look at an electrocardiogram (Figure 3).
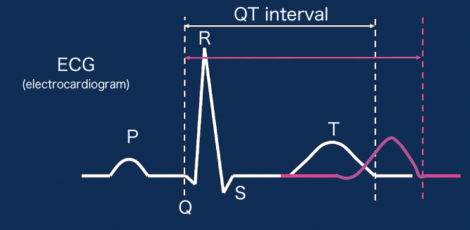
Figure 3. Sex differences in electrocardiogram wave formsThe QT interval, representing the time it takes for the heart to pump out blood, is longer in women (red line) than in men (white line). A longer QT interval could cause arrhythmia when the heart rate is high.
In an electrocardiogram, activation of the atria in the heart begins with the P wave. Then, cardiac ventricles are activated in the order of Q, R, S, and T waves. The time from the start of the Q wave to the end of the T wave represents the time from when the cardiac ventricles contract and eject blood to when they relax after the pumping. This is called the QT interval.
In 1920, British-American physiologist Henry C. Roberts (Professor, Oxford University) had reported in his article on electrocardiograms that the QT interval is longer in women than in men.
As for the reason for women’s longer QT interval, clinical research had already revealed that sex hormones were involved, so the action of estrogen (estrogenic hormone) was investigated from the 1980s to the 1990s. However, when analyses were conducted based on testosterone (male sex hormone), estrogen, and progesterone (progestational hormone), all of these sex hormones basically acted toward extending the QT interval. Therefore, they could not explain the sex difference in the QT interval.
It was around that time when we found that NO was released from sex hormone receptors in the cell membrane of the heart muscle.
We tested the action of testosterone in ventricular muscle cells of guinea pigs, and discovered that the QT interval becomes short when the testosterone in the muscle cells matches the concentration of testosterone in men’s plasma. We advocated that men’s QT interval becomes shorter, instead of women’s QT interval becoming longer, due to some signal. This article was published in one of the top circulatory system journals, Circulation. Moreover, this theory is also cited in one of the world’s most prestigious textbooks, Goodman and Gilman’s The Pharmacological Basis of Therapeutics.
Why do we focus on the QT interval? The heart contracts when activated, and then relaxes. However, if the contraction time becomes longer, it partly overlaps with the heart’s next cycle, losing time to refill the heart with blood, and could cause fatal arrhythmia.
While arrhythmia is caused by various factors, an important factor is considered to be a disorder in the heart’s rhythmical activation. For example, when the heart rate becomes fast during exercise or when one is startled by a phone ringing, arrhythmia could occur due to a spasm. Spasms are said to be more frequent in women.
We also made such simulations in joint research with U.S. scientists, and published an article this year on our finding that a rapid increase in sympathetic nerve activity induces arrhythmias more frequently in women than in men.
Arrhythmia risks of various drugs
We have gained some understanding on the mechanism of the sex differences in the electrocardiogram. However, there are aspects that are still unclear.
The risk of arrhythmia side effects must be tested in safety studies during drug development. Various drugs have been found to cause arrhythmias (long QT syndrome) when they bind to and suppress the action of the human ether-a-go-go-related gene (hERG), which encodes a subunit of an ion channel that allows potassium ions to pass.
Over the past few years, I have frequently received questions on estrogen from clinicians at academic conferences. That is because we conducted an experiment using estradiol (a type of estrogen with strong activity), and found that estradiol at low concentration slightly extended the QT interval. There is a long-standing view that estrogen, which is contained in oral birth control pills, has a risk of causing arrhythmia in women that otherwise would have no likelihood of developing arrhythmia. However, compared to the earlier mentioned action of the male sex hormone, it hardly affects the QT interval.
A further study showed that estradiol enhances the effects of drugs that suppress the action of the hERG channel and cause arrhythmia (hERG blockers). Then we found a possibility that, if a drug that is effective at a molar concentration of about 10 nM is given together with estrogen, the effects of the drug will be enhanced.
What does this mean? In joint research with U.S. experts, we asked a one who map drug binding sites from the molecular structure of proteins, using computer simulation, to carry out analysis. Then, we found that anti-arrhythmic drugs prescribed in the United States and estradiol can bind to the same hERG pockets together and this enhances the drug effects (Figure 4). In other words, we can hypothesize that anti-arrhythmic drugs and other drugs could bind to hERG pockets and amplify their effects to an unexpected level.
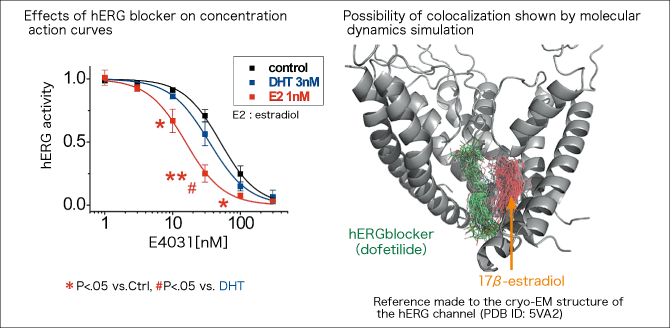
Figure 4. Interaction between estradiol and hERG blockerResearch by Professor Kurokawa, et al. revealed that estradiol at a low concentration enhances the effects of drugs that suppress the action of the hERG channel and cause arrhythmia (hERG blockers) (Kurokawa, et al., 2008 J Physiol.). By computer simulation, the team found a possibility that anti-arrhythmic drugs and estradiol can bind to the same hERG pockets together and this enhances the drug effects (Yang, Kurokawa et al., 2017 J Physiol.).
Indeed, in an experiment in which volunteers recruited in the United States were administered an hERG blocker while confirming safety, the risk of arrhythmia increased in women who were taking pills not containing anti-androgen drugs.
In the future, I would like to exhaustively screen drugs currently in use and those under development, and send out information for ensuring the appropriate use of drugs.