New types of vaccines are currently emerging, and vaccinations are being administered at a hitherto-unprecedented speed. However, greater readiness will be essential, given the risk of new pandemics and bioterrorism. It is said that using nucleic acid platforms to create mock-up vaccines in advance would enable the optimal vaccine to be produced swiftly by introducing into them a modularized antigen tailored to the pathogen concerned.
Special Feature 1 – The Past, Present, and Future of Vaccines Pursuing faster, better vaccination: The near future in the ever-evolving world of vaccines
composition by Takakazu Kawasaki
Preventive vaccines have been one of the most successful medical technologies since their invention through to the present day. The pathogens that cause infections in humans include parasites, protozoans (eukaryotic unicellular microorganisms), fungi (molds), bacteria, and viruses. The COVID-19 virus causing so many problems today is a member of the coronavirus family, which also includes the common cold, severe acute respiratory syndrome (SARS), and Middle East respiratory syndrome (MERS). The pandemic caused by this novel coronavirus has highlighted anew the importance and urgency of vaccine research.
Humans and other vertebrates are equipped with three mechanisms that defend them against pathogens:
1) Physical defenses (such as the skin and mucus)
2) Innate immunity (in the form of macrophages and the complement system that destroy pathogens)
3) Acquired immunity (in which antibodies and T cells (a type of white blood cell) work to destroy pathogens)
Tapping into the acquired immunity mechanism, vaccines are used for inoculation, to give the body a memory of the distinctive features of the virus, bacterium, or other pathogen in the form of antibodies. If the relevant pathogen enters the body of an individual who has been vaccinated, an immune reaction takes place that prevents infection or severe illness.
Various kinds of vaccine exist today
Everyone associates vaccines with British physician Edward Jenner, who carried out smallpox immunization in the late 18th century. In fact, a procedure called variolation was carried out even well before Jenner’s time in ancient China, India, and Rome, and in the Ottoman Empire in the 17th and 18th centuries. Variolation involved implanting a scab from a person infected with smallpox into a baby’s foot or blowing it up their nose.
Some people describe vaccines as attenuated pathogens. However attenuated vaccines are what were used more than 200 years ago. Various kinds of vaccine exist today, including viral vector, messenger RNA (mRNA), DNA, modified protein, and inactivated vaccines (Figure 1).
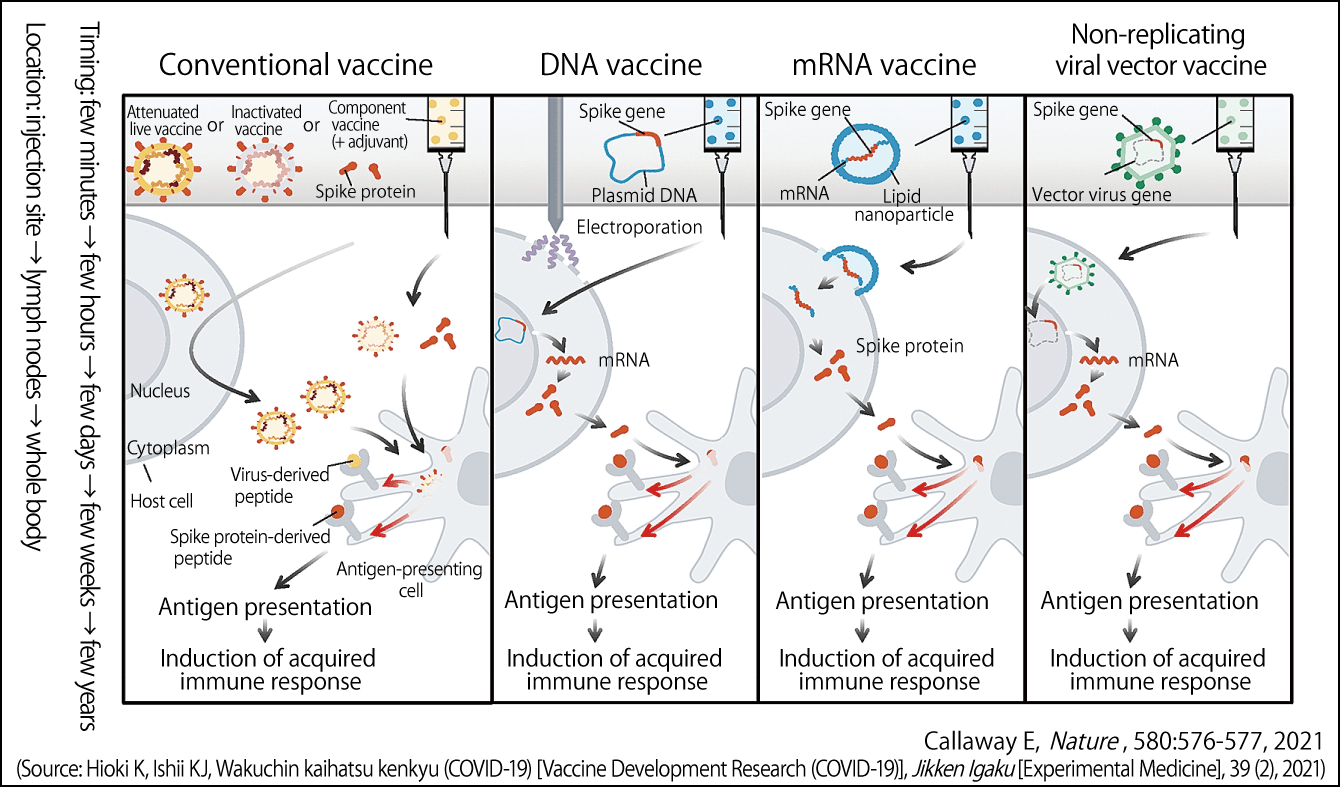
Figure 1. Main mechanisms of COVID-19 vaccinesThe mechanisms via which conventional vaccines, DNA vaccines, mRNA vaccines, and viral vector vaccines act against COVID-19. Their objective is to get immune cells to recognize the peptide derived from the virus’s spike protein as an antigen.
Conventional vaccines
In the case of conventional attenuated live vaccines and inactivated vaccines, the component vaccine is incorporated into the cells of the inoculated individual and the virus begins to proliferate in those cells. Antigen-presenting cells among the B cells and T cells identify peptides (molecules consisting of a chain of anywhere between 2 and 49 amino acids) derived from the virus that emerge from the cells as being foreign substances, triggering an acquired immune response. The effects of a vaccine are believed to last for anywhere from a few weeks to several years after vaccination.
DNA vaccines
Coronaviruses have spikes resembling a crown (“corona” is the Latin for crown) projecting from their lipid layer. DNA vaccines incorporate the gene encoding the spike into a molecule called a plasmid. When an individual is vaccinated, this plasmid enters the nucleus of a cell, and the spike protein’s DNA is transcribed into mRNA, which is in turn translated into protein. We believe that this protein is then incorporated into antigen-presenting cells and presents the antigen.
mRNA vaccines
mRNA vaccines consist of a lipid nanoparticle containing mRNA already transcribed by DNA incorporating the gene encoding the virus spike. When this mRNA enters a cell, rather than entering the nucleus, it produces the spike protein in the cytoplasm, after which antigen presentation and induction of an acquired immune response occur in the same way as with DNA vaccines. Thus, mRNA vaccines skip a step compared with DNA vaccines.
As mRNA vaccines contain fragile components, they need to be stored at very low temperatures of around -70°C.
Viral vector vaccines
The word “vector” means an animal carrying a pathogen. In viral vector vaccines, the spike gene of the virus is given residence inside a different virus. After infection, this weak virus spews out DNA into the nucleus, and the mRNA transcribed based on this creates peptides. The antigen is presented to these peptides, inducing acquired immunity.
However, if the individual already has immunity to the virus in which the spike gene is resident, a completely different immune response from that caused by DNA vaccines and mRNA vaccines may take place.
A palpably strong capability of immune induction
These vaccines are injected into the muscle cells of the arm. While the injection site swells and turns red, this is inflammation caused by innate immunity and tissue repair, and is not an immune reaction. The antigen-specific immune response that is the primary effect of a vaccine occurs in the lymph nodes, away from the injection site (Figure 2).
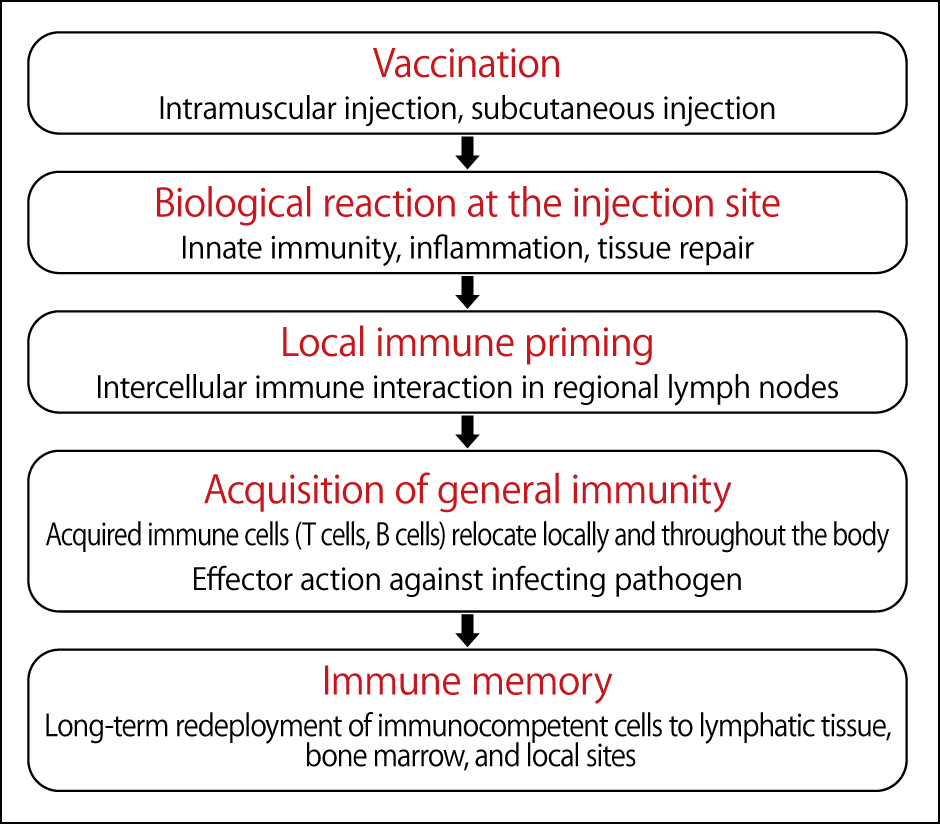
Figure 2. The process of acquiring immunity by vaccinationVaccines administered by intramuscular injection cause an antigen-specific immune response in the lymph nodes, causing antibodies to circulate throughout the body and maintaining long-term immunity.
Antibodies subsequently circulate throughout the body, providing long-term immunity. However, there are still many things we do not know about immunity, such as how T cells and B cells circulate throughout the body and prepare for attack by various pathogens, and whether this occurs in the bone marrow or locally.
The Japanese government approved the mRNA COVID-19 vaccine produced by Pfizer of the U.S. and German company BioNTech, and vaccinations began in February. Vaccinations using the mRNA vaccine manufactured by Moderna of the U.S. and British pharmaceutical company AstraZeneca’s viral vector vaccine are due to begin in May.
So what are the differences between these vaccines?
Speed of production: Live vaccines and inactivated vaccines use cells from animals and hens’ eggs, so they take about half a year to make. DNA and mRNA vaccines, on the other hand, can be produced in large quantities very quickly.
Immune induction capability: That is to say, vaccine effectiveness —— live vaccines are very good, because they only imitate the viruses that cause disease, but one cannot say that the risk of catching the disease is zero.
The capabilities of inactivated vaccines, DNA vaccines, and mRNA vaccines to induce immunity vary widely. Of these, mRNA vaccines achieve a palpably powerful immunity of a kind hitherto unseen.
Safety: The safety of DNA vaccines has been established, but mRNA vaccines are being used for the first time and the safety of vaccines produced in some countries has not been guaranteed, so question marks remain in some areas.
Development based on a nucleic acid platform
From 1996, I myself was involved in the development of DNA vaccines and adjuvants (substances that enhance the effects of vaccines) when I was a visiting scientist at the Food and Drug Administration (FDA) and Center for Biologics Evaluation and Research (CBER) of the Department of Health and Human Services (HHS) in the U.S. I was also fortunate enough to have the opportunity to review them as an IND (investigational new drug) reviewer.
I returned to Japan in 2003, and then in 2010, at the National Institute of Biomedical Innovation (now the National Institutes of Biomedical Innovation, Health and Nutrition [NIBIOHN]), I began working on production of an mRNA vaccine for MERS and was involved in the development of a vaccine for SARS. Around that time, I was also involved in research and development focused on new DNA vaccines and adjuvants. In 2006, at Osaka University, I began to shed light on the mechanisms of DNA vaccines.
Experiencing the 9/11 terrorist attacks in 2001, while I was studying in the U.S., I am painfully aware of the need for vaccines that can be produced and deployed immediately against bioterrorism and outbreaks of manmade infectious diseases, so I have continued working on vaccine development.
In 2016, I embarked on a joint research project with Dr. Yasuhiro Yasutomi, Director of the Tsukuba Primate Research Center of NIBIOHN, and a company, aimed at rapid vaccine development based on nucleic acid vaccine platforms (DNA and mRNA) and nucleic acid adjuvants as an emergency infection control measure. This led to the launch of the mock-up vaccine project.
Unfortunately, this project was put on ice in 2018 after the government cut the budget for clinical trials. However, after I moved to the Institute of Medical Science at the University of Tokyo, I resumed my research and have now embarked on a project entitled “Study on the control of a novel coronavirus (2019-nCoV)” in partnership with two colleagues from the institute, Professor Yoshihiro Kawaoka and Professor Hiroshi Yotsuyanagi.
Creating a vaccine takes 10 to 20 years and requires numerous development staff, including anywhere from several dozen to several hundred of vaccine researchers, along with up to 100,000 clinical trial participants in the biggest studies, and a budget in excess of ¥100 billion. The reason why it was possible to develop the vaccines for this pandemic in less than a year in such countries as the U.S., the UK, and China is that as much as ¥1 trillion was invested, and the preclinical studies, the clinical studies, and the Phase 1, 2, and 3 trials were able to be conducted in parallel (Figure 3). In contrast, Japan decided to contribute ¥10 billion to vaccine development —— just one-hundredth of the sum spent by Western countries on development. Due to the problems caused by COVID-19, development staff were not able to get together.
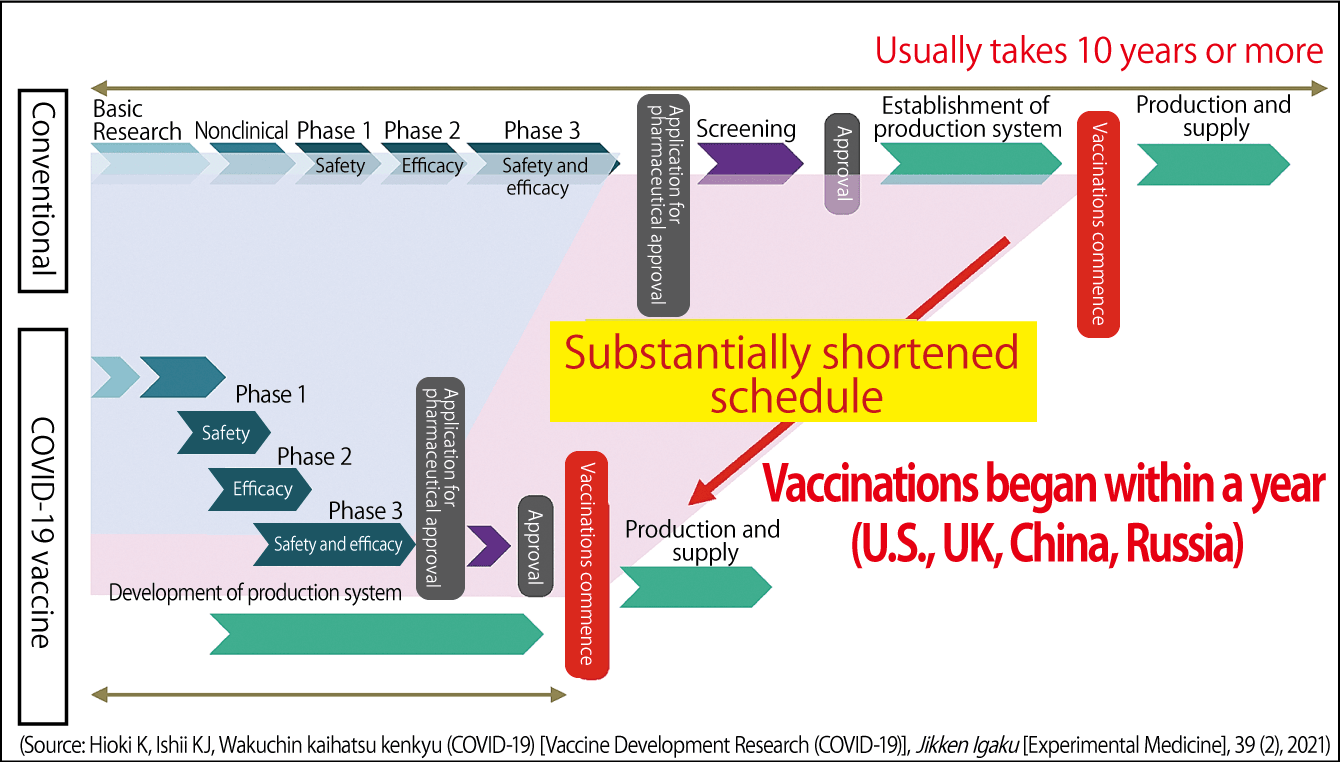
Figure 3. COVID-19 vaccine development scheduleCreating vaccines requires huge sums of money and takes upward of 10 years, because the steps from basic research through to the numerous trials are carried out in series. In response to the recent pandemic, the U.S., the UK, and China spent ¥1 trillion on testing and carried out the trials in parallel, enabling vaccination to begin within a year.
On hearing that we are developing a COVID-19 vaccine, some people ask, “Is there any point, since we’re so far behind the West?” However, in Asia in particular, Japanese pharmaceuticals have a brand image of being safe and reliable. Some of my friends from other parts of Asia have told me that if Japan successfully developed its own vaccine, they would import it to vaccinate their own people.
Moreover, vaccine development is affected to a great degree by foreign policy, national defense, economics, and industry, and is the key to public health and safety, which will ultimately ensure that citizens are safe.
I personally believe that it would be wise to ensure readiness by developing modularized vaccines that can be assembled for each pathogen, combining the separate vaccine components of antigens, adjuvants, and delivery systems like the parts of a car. We plan to prepare a mock-up vaccine that combines these parts (Figure 4).
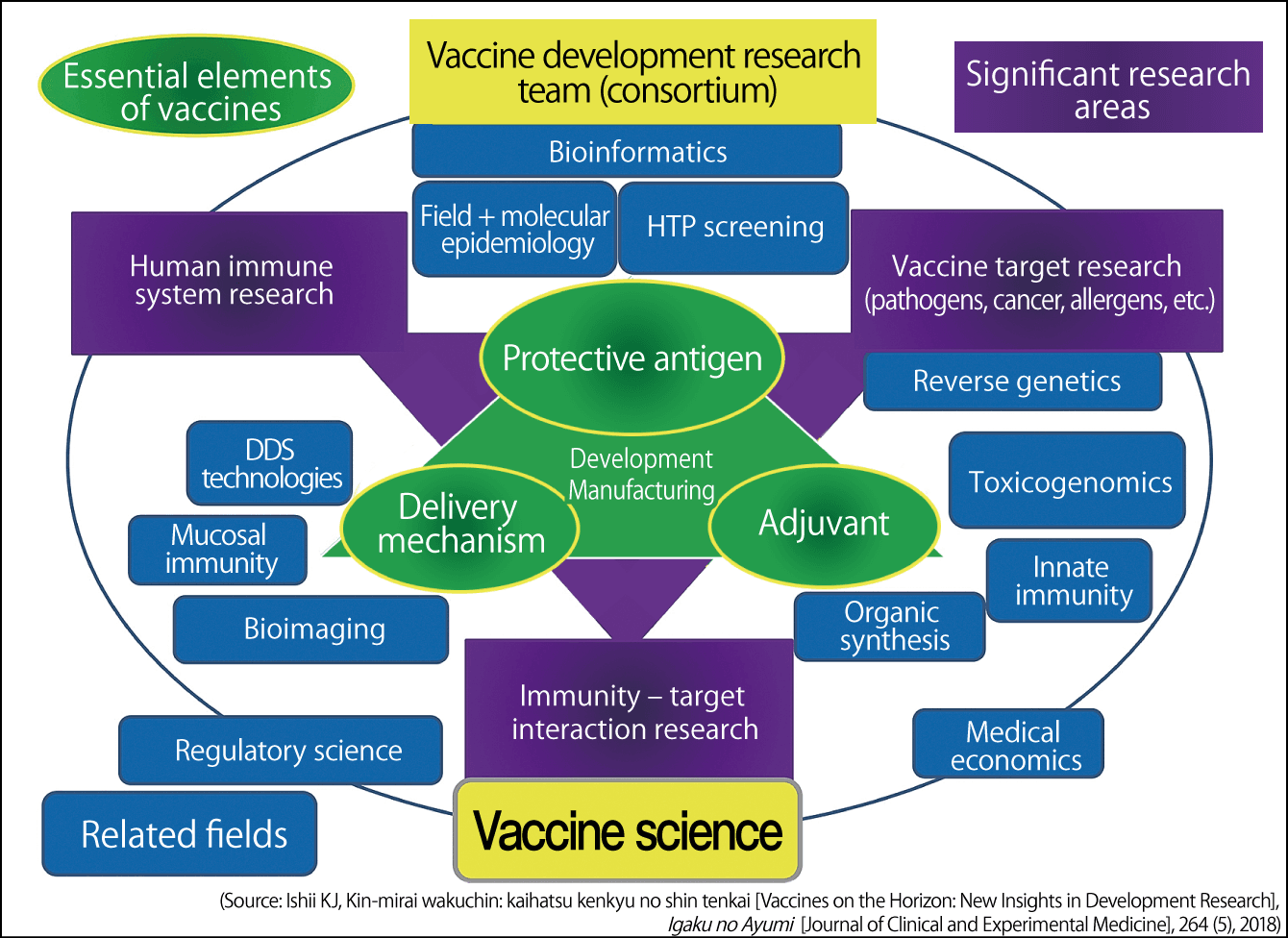
Figure 4. Illustration of new vaccine development researchVaccine development requires the mobilization of science and technology by dedicated teams focused on antigens, adjuvants, and delivery systems. Efficient vaccine development would become possible if antigens, adjuvants, and delivery systems could be modularized and combined as needed.
Mock-up vaccines offer major advantages.
1) Even in situations where we do not know which infections will occur when, if we made mock-up vaccines that combined mock-up antigens with a number of adjuvants, we would be able to produce the optimal vaccine immediately. They would also enable us to counter infectious diseases other than COVID-19.
2) If we knew the base sequence or amino acid sequence of the pathogen’s antigen, we would be able to create the DNA or mRNA literally overnight.
3) If we had the nucleic acid of the DNA or mRNA, we would be able to set up lots of small vaccine manufacturing plants nationwide. There would be no need for big manufacturing tanks, and it would allow production to take place on a small scale, enabling us to respond swiftly to outbreaks of infectious diseases.
We have already completed our mock-up vaccine experiments. We obtained very good results from these experiments and have confirmed immunoreactivity in humans in the test tube. We have filed a patent application and have already prepared an article.
We plan to start the Phase 1 sponsor-initiated clinical trial before the end of FY2021.