2023 saw the first use in clinical practice of anti-amyloid drugs, which inhibit the worsening of Alzheimer’s disease symptoms by acting on the substance that causes the disease. While the move was reported as offering a long-awaited ray of hope in regard to dementia, for which there is no effective cure, one has to consider the fact that these drugs are indicated solely for mild, early-stage Alzheimer’s disease. Accordingly, scientists are making haste to develop biomarkers that will enable the disease to be diagnosed accurately at an early stage. In particular, hopes are high that an efficient, noninvasive, and cheap blood biomarker could be put to widespread use in routine medical checkups and the like.
Special Feature 1 – The Mechanisms and Functions of Blood Alzheimer’s disease biomarkers that facilitate early diagnosis of dementia
composition by Yuko Watanabe
Japan is becoming a super-aging society. Figures published by the Ministry of Health, Labour and Welfare in 2012 estimate that the number of dementia patients in Japan will break the 7 million barrier in 2025, which means that one in five elderly people aged 65 or above will suffer from dementia. The primary diseases of dementia include a diverse array of conditions, with Alzheimer’s disease believed to account for 60-70% of these, and the prevalence rate increases markedly as people age (Figure 1).
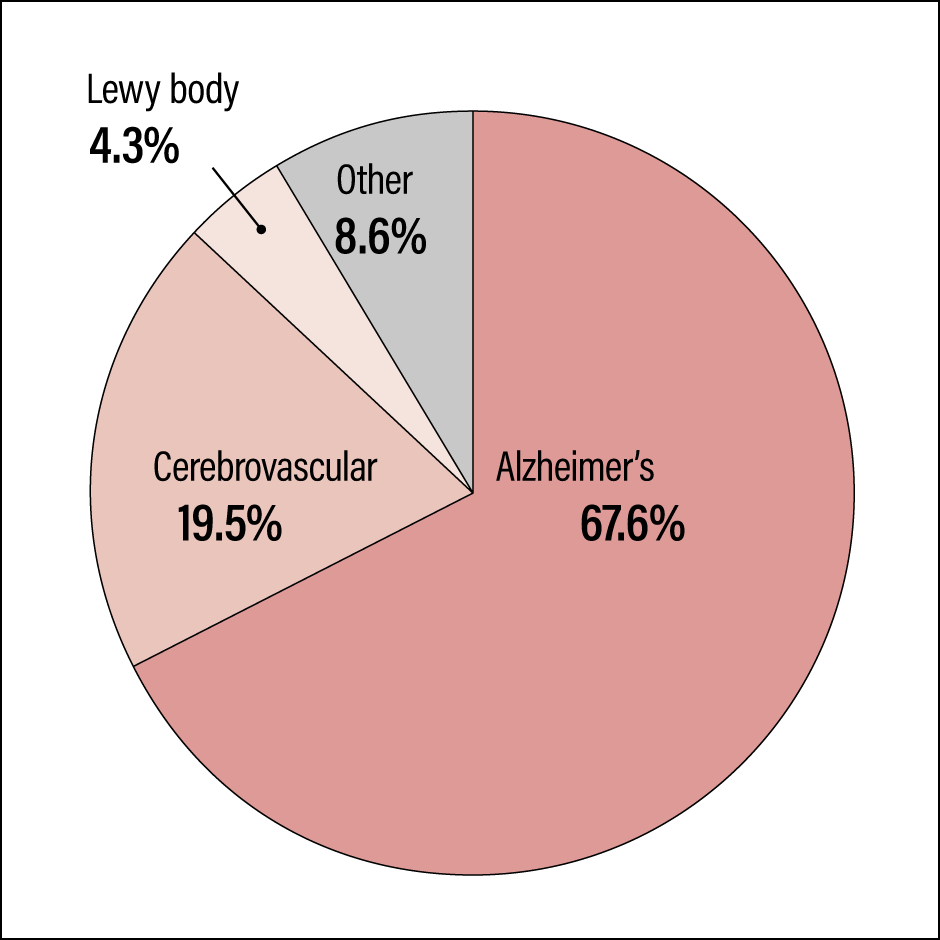 Source: Incidence Rate of Dementia in the Urban Area, and Response to Living Functional Disability Caused by Dementia (Comprehensive Research Project on Measures against Dementia, Health Labour Sciences Research Grants)
Source: Incidence Rate of Dementia in the Urban Area, and Response to Living Functional Disability Caused by Dementia (Comprehensive Research Project on Measures against Dementia, Health Labour Sciences Research Grants)
Figure 1. Main types of dementia and share of all casesWhile Alzheimer’s disease is often the primary cause of dementia, accurate diagnosis is complicated by the fact that in some cases, it coexists with conditions such as cerebrovascular dementia and dementia with Lewy bodies. This makes it difficult to determine the optimal timing for the use of disease modifying therapies.
Progressive cerebral atrophy caused by neuronal damage
Alzheimer’s disease is a neurodegenerative disease characterized by senile plaque amyloid consisting of amyloid beta-protein (Aβ) that accumulates outside brain parenchymal cells, along with neurofibrillary tangles consisting of tau protein that clumps and builds up within neurons, causing progressive cerebral atrophy due to neuronal damage. It is thought that Aβ amyloid pathology begins to clump and accumulate in the brain about 20 years before the onset of dementia; once this induces the clumping and buildup of phosphorylation tau protein (p-tau), widespread neuronal damage (neurodegeneration) occurs, which causes atrophy of the cerebrum and the onset of Alzheimer’s disease.
Aβ is present even in the blood of healthy individuals, and p-tau also accumulates in the medial temporal lobe in the course of the normal aging process, albeit only in very small quantities. However, scientists still have not unraveled the reason why Aβ and p-tau clump and accumulate. They believe that Aβ amyloid pathology, which emerges in the earliest stages of Alzheimer’s disease, does not on its own cause severe neuronal damage; rather, the induction and buildup of p-tau by Aβ amyloid pathology causes neurodegeneration, which leads to the progression of dementia.
A turning point in the treatment of dementia was reached in 2023, when lecanemab began to be used in clinical practice. Rather than being a symptomatic therapy, lecanemab is a disease modifying therapy (DMT) developed in Japan that inhibits the onset and progression of Alzheimer’s disease by acting on the substance that causes the disease.
As an anti-amyloid drug that selectively binds with Aβ and removes it from the brain, the DMT lecanemab can be expected to inhibit the onset and progression of Alzheimer’s disease, thereby preventing symptoms from worsening. The onset of Alzheimer’s disease occurs after the buildup of Aβ over many years, causing a progressive decline in cognitive functions. One feature of lecanemab is that it is indicated solely for people with early-stage Alzheimer’s disease —— that is to say, those who have either mild cognitive impairment (MCI) prior to onset or mild dementia only.
However, one frequently encounters patients in whom the diagnosis of Alzheimer’s disease can be difficult, even for highly experienced neurologists, particularly in the case of early-stage Alzheimer’s disease. Another factor complicating the accuracy of the clinical diagnosis of Alzheimer’s disease is the fact that in people aged 70 and above, one hardly ever sees symptoms due to Alzheimer’s alone; cerebrovascular dementia, dementia with Lewy bodies, and various other neurodegenerative pathologies are mixed pathologies that coexist with varying frequency in different patients.
Diagnosing the extent of neurodegenerative progression
It is thought that by the stage in which cognitive impairment appears, Aβ has already reached saturation and tau pathology is progressing. However, the optimal target for lecanemab should be someone in the very early stage, in which tau pathology has not progressed, but assessing the degree of progression to identify the timing of administration is not easy.
With the emergence of this DMT, the establishment of biomarkers (BMs) to accurately diagnose Alzheimer’s disease patients has become a matter of urgency, alongside efforts to shed light on Alzheimer’s disease pathology, and the development of precise diagnosis and therapies.
A BM is defined by a characteristic that can be objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes or pharmacological responses to a therapeutic intervention. Examples of BMs indicative of normal biological processes include blood glucose level, which indicates the level of satiety after eating a meal, and electroencephalograms, which are used for evaluating sleep stages and the like. Looking at pathogenic processes and pharmacological responses, levels of prostate-specific antigen (PSA) in the blood can be used as a prostate cancer tumor marker to identify the onset of the disease, while MRI images of the brain can be used to determine whether brain tissue has been protected by the administration of a thrombolytic agent in the acute phase of a stroke.
An Alzheimer’s disease BM needs to be able to be used to objectively measure and evaluate senile plaques (of which Aβ is the main component) and neurofibrillary tangles (consisting of p-tau) that have formed in the cerebrum, which are substantive characteristics of neuropathology. For example, to facilitate the use of an anti-amyloid drug like lecanemab, it is crucial that the BM should be capable of correctly diagnosing the extent to which Aβ and p-tau accumulation and neurodegeneration have progressed.
The ATN-BM system was proposed in 2018 as a framework for using biomarkers to diagnose and stratify the stages of brain pathology in Alzheimer’s disease, and has now become the international standard. ATN-BM rates as either + or – the results for BMs that reflect the three characteristics of Alzheimer’s disease: Aβ accumulation (A), p-tau accumulation (T), and neurodegeneration (N). They are then combined to determine whether the patient has Alzheimer’s disease or another neurodegenerative disease, and if the former, the stage, which represents the extent of Alzheimer’s progression.
The tests used in the ATN-BM system are imaging BMs in the form of positron emission tomography (PET) and MRI examinations, and a fluid BM, in the form of a cerebrospinal fluid test (cerebrospinal BM). Amyloid PET and tau PET examinations provide clear images of the extent of A and T buildup, and are the most precise forms of BM —— so precise, in fact, that they can be a substitute for pathologic diagnosis. The utility of the cerebrospinal BM has been established following verification that it can detect A, T, and N with a high level of sensitivity and specificity. N is also evaluated by using MRI to determine the degree of lesion damage.
However, the imaging and cerebrospinal BMs used in ATN-BM do pose some challenges. Problems with PET examinations include their low efficiency, given that only three examinations can be carried out at each facility per day; the fact that they are expensive, costing in the region of 150,000-300,000 yen; and the limited number of facilities capable of carrying out PET examinations. At the same time, with a disease as common as Alzheimer’s, the cerebrospinal BM positioned as a screening test entails such problems as the invasiveness of the lumbar puncture procedure for the patient, and the fact that it has to be carried out by a specialist physician, which means that it is neither easy nor universal. As such, the cerebrospinal BM is not a suitable complement to imaging BMs.
In addition to imaging and cerebrospinal BMs, attention is focusing on blood BMs as a means of achieving the early-stage and stratified diagnosis of Alzheimer’s disease. A blood BM would be efficient, less invasive, lower cost, easy, and universal, while allowing for quantitative evaluation.
Blood BMs that evaluate the asymptomatic preclinical stage
At the 2023 Alzheimer’s Association International Conference, the incorporation of blood BMs into the diagnostic criteria for Alzheimer’s disease was accepted, in light of recent progress in blood BM research and development. The race to develop and establish blood BMs for ATN-BM, too, is intensifying both within Japan and overseas.
In order to diagnose and treat Alzheimer’s disease, we need blood BMs to enable us to evaluate the preclinical stage when there are no clinical symptoms, and the very early stage that represents the optimal time to begin treatment. As a board-certified neurologist, I have been engaged for many years in the research and development of BMs that, through the quantification of relevant disease-specific molecules in cerebrospinal fluid and blood, facilitate the simple and objective diagnosis and assessment of neurodegenerative diseases that are difficult to diagnose accurately and whose severity is hard to assess using clinical symptoms alone. These diseases include Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis (ALS), and Down syndrome. So far, we have succeeded in developing the world’s first quantification system that detects abnormal levels of proteins, such as alpha-synuclein in the case of Parkinson’s disease and TDP-43 for ALS, thereby serving as cerebrospinal BMs for patients.
Furthermore, in September 2017, we successfully developed the world’s first quantification system that employs single molecular array (Simoa) —— an ultrasensitive digital assay technology offering 1,000 times the conventional level of detection sensitivity —— to detect p-tau181 (tau phosphorylated at threonine, the 181st amino acid on a tau molecule), which is found in trace amounts in the blood, but had until then been difficult or impossible to detect (Figure 2).
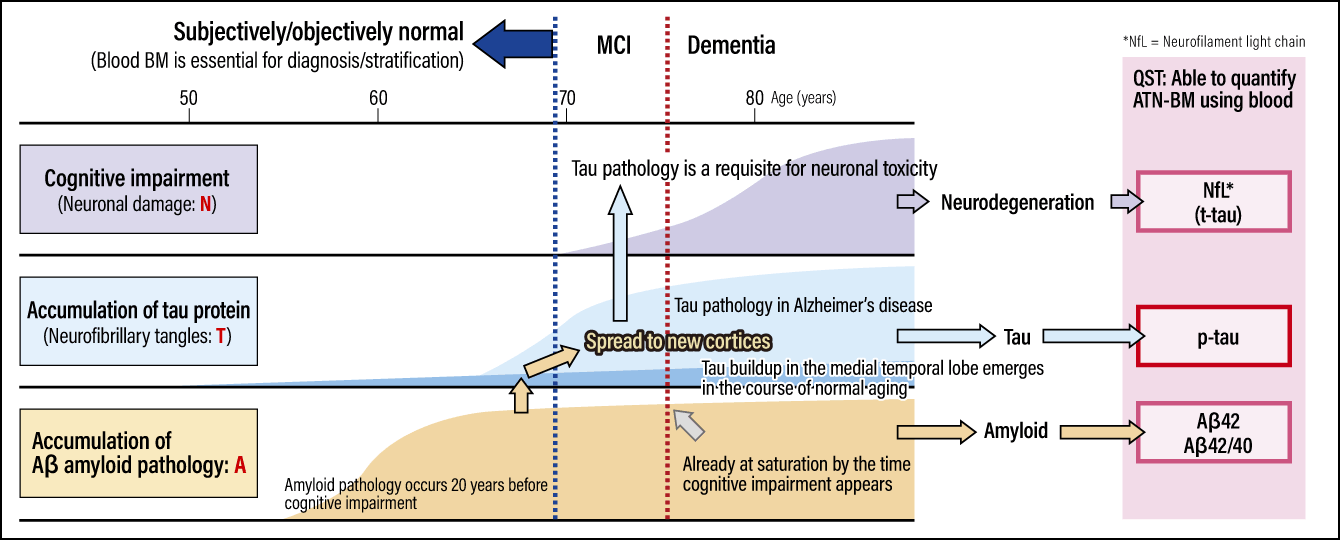
Figure 2. Alzheimer’s disease brain pathology over time and ATN-BMThe buildup of tau protein begins around 5-10 years after the accumulation of Aβ amyloid pathology, which starts around 20 years before the onset of dementia. ATN-BM has been proposed as a means of objectively identifying the progression of Alzheimer’s disease in the brain, including during the very early stage, when brain pathology has begun, even though cognitive impairment has not yet developed. Proposed as the international standard in 2018, ATN-BM uses imaging and cerebrospinal BMs, but at QST, all of ATN-BM can be quantified using blood.
When we used this system to quantify p-tau levels in the blood of actual patients, we found that Alzheimer’s patients had statistically significantly higher blood p-tau levels than individuals in the normal control group. Furthermore, when we compared the results for a cohort of patients with Down syndrome —— a condition known to be associated with the emergence in the cerebrum of neurofibrillary tangles consisting of p-tau once individuals are in their 40s —— with those of another control group, we found that the blood p-tau levels measured with this system were a BM reflecting the emergence of neurofibrillary tangles, which are most closely associated with cognitive impairment in patients with Alzheimer’s disease and Down syndrome. Accordingly, we were able to report that blood p-tau is a useful blood BM for the diagnosis of Alzheimer’s disease.
The fluid BM in the ATN-BM system proposed as the international standard in 2018 refers to the cerebrospinal BM. Today, however, numerous research teams, including ours, are reporting that it is possible to quantify all relevant molecules in blood that are specific to Alzheimer’s disease and can be used in ATN-BM, and a steady stream of reports endorsing the utility of these BMs is also appearing on the international stage. In the area of relevant molecular species specific to diseases, too, research into blood BMs is surging further, with verification and discussion efforts progressing in greater depth following fresh discoveries of molecular species that provide a purer reflection of each ATN indicator.
The National Institutes for Quantum Science and Technology (QST), which I joined in 2020, is leading the world in the development of PET probes (compounds used to visualize the movement of various molecules within the body) for degenerative diseases.
In addition, our research team is eagerly working on blood BM development, including the successful detection of all ATN-BM using blood BMs. Research into the quantification of other disease-specific substances using blood BMs is also progressing, and we have achieved a number of successes. Biochemical diagnosis of TDP-43 —— the causative protein of ALS and frontotemporal lobar degeneration —— had previously been impossible, but in 2021, our research team became the only one in the world to succeed in quantifying the protein in blood and cerebrospinal fluid.
Comprehensive diagnosis of brain pathology in dementing illness
Looking at the potential of the blood BMs we have developed and the possibilities for using them to diagnose brain pathologies, the first thing I would say is that blood BMs offer a highly efficient, noninvasive, cheap means of reaching an objective, accurate diagnosis of dementing illness and neuropsychiatric disorders, and of evaluating their stratification (severity). As such, I believe they can be used as a first-stage examination or screening test (followed by definitive diagnosis using PET).
Second, I believe they could be used to facilitate treatment if they were employed as a rapid screening test in the health checkups organized by local governments and the like for large numbers of elderly people, before conducting complex neuropsychological examinations and assessments of motor symptoms. For example, if a blood test at the age of 55 or so confirmed issues in ATN-BM, the physician might decide to start treatment at an early stage, saying, “You haven’ t developed dementia yet, but your p-tau level is high, so Aβ may well be building up. I’ll give you an anti-amyloid drug to reduce Aβ.” If Aβ declines to near zero after a year of taking the drug, it will take 3-4 years for Aβ to build up again, so the patient can lead a healthy lifestyle for a few years and have another blood test to check levels 3-4 years later. That is the kind of treatment method I have in mind. My hope is that this kind of bespoke medical care tailored to the individual patient’s brain pathology will be achievable in the not-so-distant future.
Third, if blood BMs for ATN-BM evolve to become more reliable and simpler, we can expect the development of dementia DMTs to progress at an accelerated pace.
Turning now to tasks going forward, first of all, I think there is a need for a multi-item BM that goes beyond the current ATN framework to incorporate a variety of other disease-related substances as a set, such as alpha-synuclein, TDP-43, and microglia, in order to facilitate not only the diagnosis of Alzheimer’s disease, but also the comprehensive diagnosis of brain pathology in dementing illness. Second, we need a BM capable of quantitative evaluation —— as opposed to the binary + or – judgment currently used in ATN-BM —— that can be used in future clinical trials of DMTs for the accurate identification of eligible patients and determination of the effects of treatment. Third, I would like to conduct verification using a large cohort, in order to achieve the standardization of blood BMs.
Currently, at QST, we are developing mutually facilitative imaging and fluid BMs (primarily blood BMs, in the latter case), ultimately aiming for the establishment and clinical application of a multi-item integrated BM (Figure 3).
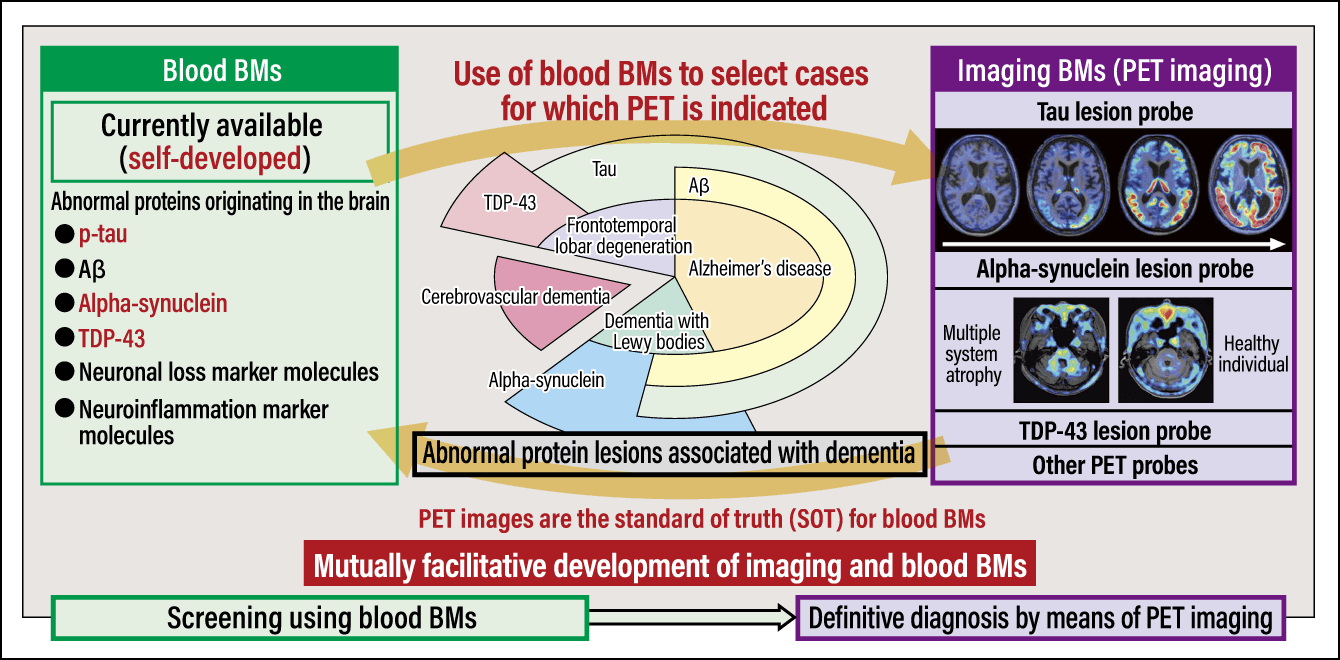
Figure 3. Development of imaging and blood BM systems underway at QSTThe research team at QST is undertaking mutually facilitative development of imaging BMs (PET imaging) and blood BMs, and has discovered self-developed relevant molecular species specific to diseases, making it possible to quantify them in the blood. In the future, the research team aims to integrate multi-item imaging and blood BMs, and to develop a unified comprehensive diagnosis and stratification system for dementia.
I am confident that this system will be capable of delivering innovative progress in every aspect of the diagnostic process for dementia, from routine medical care to clinical research.