While chronic kidney disease is highly prevalent in cats, its precise cause is not known and scientists had believed it to be incurable. However, it has now been discovered that a protein called apoptosis inhibitor of macrophage (AIM) holds the key to a remedy for this disease. Functioning in many animals, AIM’s role is to make phagocytic cells called macrophages consume dead cells and other waste in the body. However, for some reason, the cat family is the only one in which AIM is consistently inactive, with the result that waste builds up in the kidneys, causing kidney disease. Administering AIM causes macrophages to consume this waste and improves kidney function.
Special Feature 1 – Living with Pets Could the macrophage-derived protein AIM save cats from kidney disease?
composition by Takeaki Kikuchi
illustration by Rokuhisa Chino
As they age, almost all cats develop kidney disease, from which they eventually die. Just as in humans, kidney disease is incurable in cats. However, with the development and clinical trials of a drug to treat feline kidney disease now underway, an application for the drug’s approval could, if all goes well, be submitted during the next fiscal year, or the one after that at the latest.
AIM is constitutively inactive in cats
The substance used in this therapeutic drug is a protein called AIM. This protein is found in the bodies of both humans and animals, where it serves to prevent the progression of disease. However, while it is also present in cats, we know that AIM remains inactive in their bodies, with the result that they eventually die from kidney disease. The remedy we have been dreaming of involves seeking to cure kidney disease by injecting AIM or activating AIM in the body.
AIM is a molecule that I discovered in 1999. I had previously discovered that a molecule called HLA-DM operates in the selection of T lymphocyte (T cell); while working at the Basel Institute for Immunology in Switzerland, I found a new molecule with a similar shape to HLA-DM, and embarked on research to investigate whether it was involved in T cell function. However, after six months of work, I learned that the new protein I had discovered had no connection to T cells, but appeared to prolong the life of macrophages (phagocytic immune cells that consume pathogens that have invaded peripheral tissue) in the body. I named this protein “apoptosis inhibitor of macrophage,” or AIM for short.
Although I had discovered it, its function remained a mystery. AIM is present in large quantities in the blood, but I had no idea what role it served in the body. As I had, up to that point, always been engaged in research on the front line of immunology, and as it was a protein produced by immune cells, namely macrophages, I unquestioningly assumed that it was a molecule that affected the immune system. I produced knockout mice without AIM and compared them with normal mice, but there was no difference whatsoever between them in terms of T cell function or the function of any other cells in their immune systems.
It was only after I moved to the University of Texas in the U.S. that I was able to ascertain the function of AIM. Said to be the world’s top center for research into obesity and cholesterol, the University of Texas had some eminent researchers. One day, I was standing chatting to Dr. Joseph L. Goldstein, who had been awarded the Nobel Prize in Physiology or Medicine. He said to me, “It seems as though you’ve been approaching your research into AIM entirely from the perspective of immunity. Why don’t you try doing some experiments focused on obesity and cholesterol as well?”
When I then tried making AIM-free knockout mice put on weight, I discovered that they were more prone to worse obesity and fatty liver than overweight mice with the AIM protein. I learned that there were also large accumulations of AIM in atherosclerotic plaques. I had found out that, rather than being connected to immunity, AIM appeared to be a molecule that somehow controls lifestyle diseases related to fat. I then discovered that AIM even had the effect of noticeably curbing the onset of liver cancer, which develops from fatty liver.
I subsequently sought advice from kidney researchers and used laboratory mice to investigate the relationship of AIM to kidney disease, whereupon I found that, when the mice developed acute kidney injury, those in which AIM did not function rapidly deteriorated and most died of kidney failure. However, when I injected them with AIM, their kidney impairment swiftly improved and they recovered. This meant that the molecule was not solely related to fat. Understanding that there was a more fundamental mechanism, I found out that AIM’s basic function was actually to clean up waste in the body.
Macrophages use AIM as a marker when consuming waste
Neither lifestyle diseases such as obesity and fatty liver, nor kidney disease, nor cancer are caused by viruses or other pathogens that have come from outside the body. If waste such as the remains of dead cells and degraded proteins, and excess fat are not properly removed from the body, they will cause chronic inflammation and diseases will continually deteriorate. In the case of cancer, cells that had originally been functioning within our own bodies become cancerous by chance, and if those cancer cells are not removed, they will continue to proliferate and form tumors. This, too, could be described as a disease in which waste that should be removed gradually accumulates in the body.
While I was researching kidney disease, it became clear that AIM was a very important protein that clears out various waste generated within the body and halts the progression of a range of diseases. I eventually succeeded in shedding light on this mechanism. AIM’s function is making macrophages consume waste.
Under normal circumstances, 1 ml of blood contains around 5 µg of AIM, which is bonded to an antibody called immunoglobulin M (IgM) (Figure 1). When an individual is healthy, AIM is in an inactivated state as a result of bonding with IgM, but when waste builds up in the body, AIM separates from IgM and bonds with the waste. It is not the case that the AIM itself has the effect of breaking down or dissolving the waste when it bonds with it. Rather, the bonded AIM serves as a marker that attracts macrophages, which consume and remove the waste along with the AIM. AIM usually bonds with IgM, but as IgM is a very large molecule, macrophages cannot consume it.
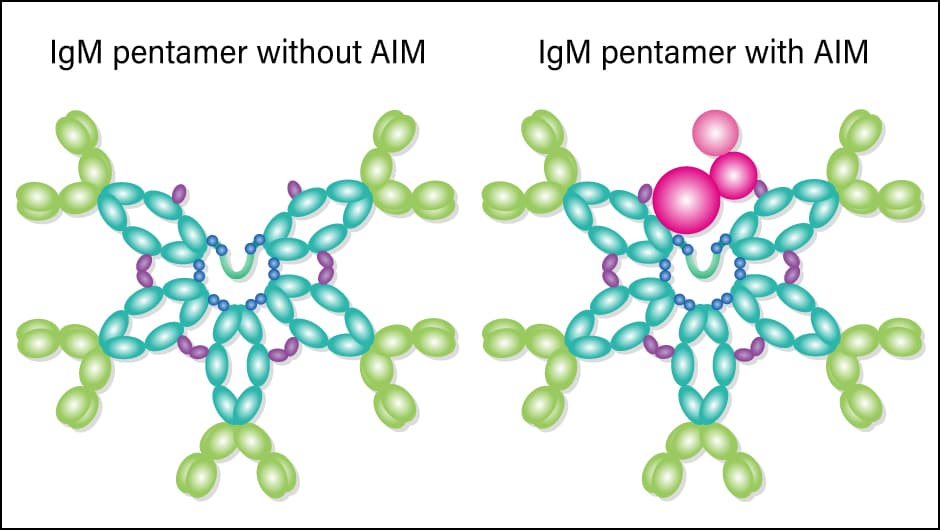
Figure 1. AIM and IgM pentamerRather than being present in isolation in the blood, AIM bonds with an IgM pentamer, which consists of five IgM molecules. The shape of an IgM pentamer looks as though it has made space to incorporate an AIM protein.
One could liken AIM to the stickers put on large items of waste in Japan to mark them as needing collection. Affixing the sticker when putting out large items of waste informs the workers on the garbage truck that the item is waste that needs to be collected. The same process happens within the body. The adhesive surface of a large waste sticker is usually attached to a backing paper, from which it is peeled off and affixed to the item of waste for collection. Similarly, AIM is usually attached to backing paper in the form of IgM, but once it finds some waste, it peels itself off the backing paper and affixes itself to the waste. The macrophages —— akin to a garbage truck —— clear up only the waste to which the AIM “sticker” has been affixed.
Allow me to explain the process via which AIM becomes affixed to the waste. Usually, the surfaces of normal cells and proteins have a positive or neutral charge, while the underside of a cell or interior of a protein has a negative charge. When a cell dies or a protein becomes degraded, the negatively charged side comes to the surface. It is to this part that the positively charged surface on the underside of AIM (the C-terminal end) attaches.
A buildup of waste causes an “incurable disease”
In the second stage, an amino acid called cysteine becomes active. Normally, there are even numbers of cysteine molecules in proteins, with pairs of cysteine molecules forming a bridge by means of a strong bond called a disulfide bond. For example, if there are eight cysteine molecules in a protein, the four cysteine pairs serve to solidify the shape of the protein. However, AIM is very unusual in having an odd number of cysteine molecules. This leaves a single cysteine molecule that cannot form a bridge, which protrudes from the surface of the AIM protein. At the same time, in degraded proteins —— that is to say, waste —— the disulfide bonds within the protein have broken, and single cysteine molecules appear on the surface. In the remains of dead cells, too, the proteins on their surface break up, causing single cysteine molecules to be exposed. The cysteine molecule on the AIM surface forms a disulfide bond with the exposed cysteine molecule in the waste, resulting in a strong and stable bond between the AIM protein and the waste.
If there are not enough AIM proteins to deal with the waste, unconsumed waste (waste that was not collected because a sticker had not been affixed to it) will gradually build up and, before one knows it, the inside of the body will be filled with waste, like a hoarder’s house. One could probably describe this as the basic mechanism underlying fatty liver, kidney disease, cancer, and incurable diseases such as Alzheimer’s disease.
I had been researching AIM with a view to curing kidney disease in humans, but I happened to have the opportunity to talk to a veterinarian. He told me, “Almost all cats develop kidney disease. The rate of death from kidney disease in dogs is almost the same as in humans, but the rate in cats is remarkably high. While some cats do die of cancer, most of those cats also have kidney disease.” The fact that most cats develop kidney disease suggests that feline kidney disease is a congenital or hereditary disease.
When I investigated further, just in case, I discovered a highly interesting problem. Feline AIM has distinctive differences from AIM in humans, dogs, mice, and the like in terms of its amino acid sequence, and is very strongly bonded to IgM. Because they are so strongly attached, the AIM protein cannot peel off from the IgM and bond with waste when it appears in the kidneys. Accordingly, waste builds up without the AIM being attached to it, causing the kidneys to become chronically impaired —— in other words, the individual develops chronic kidney disease (Figure 2).
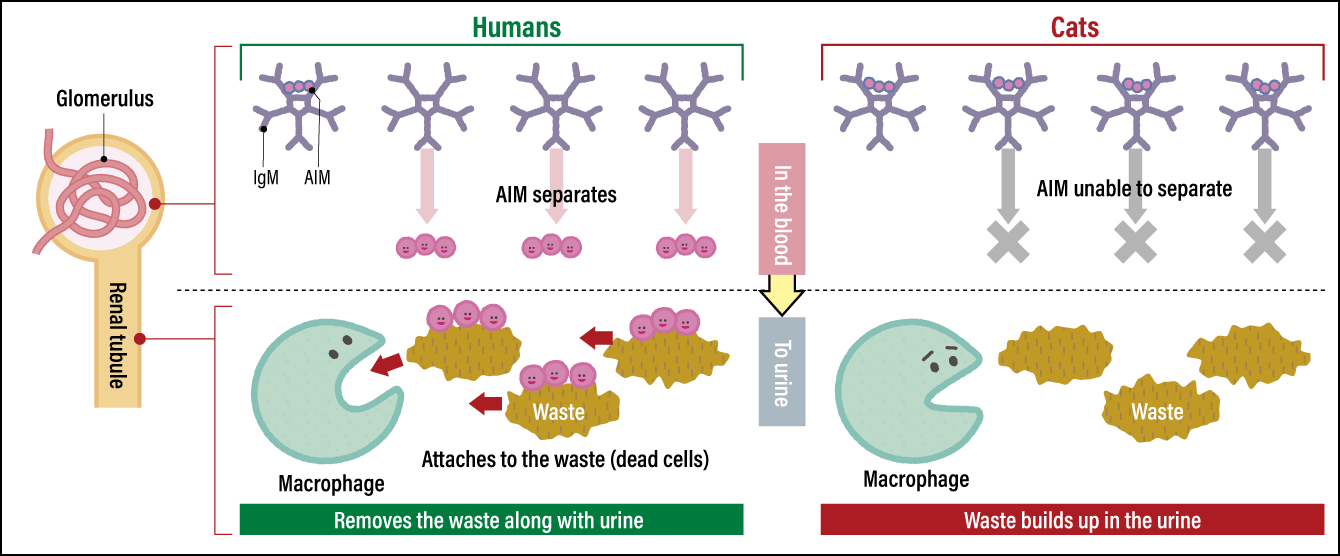
Figure 2. How AIM functions (differences between humans and cats)In the case of humans, the AIM protein that was bonded to IgM in the blood separates off and moves into urine. It then bonds with waste (dead cells and the like) blocking the renal tubules. Macrophages clear the waste by eating it. As feline AIM does not separate from IgM, waste in the urine cannot be cleared and kidney function declines as a result of the waste that has continued to build up.
If kidney disease develops because AIM is not functioning, it stands to reason that supplementing AIM should cure kidney disease. In the case of cats in which the disease has progressed because a large amount of waste has already built up in the kidneys, it is necessary to achieve a powerful waste clearance effect by injecting them with AIM (Figure 3). On the other hand, if not that much waste has accumulated and the disease is still at a comparatively early stage, it might, I thought, be possible to clear the waste via the activation of the AIM still bonded to IgM by forcibly tearing it off from the IgM. In the process of my research, I confirmed that the amino acid L-cysteine has the function of peeling AIM off from IgM at a certain level. Accordingly, I worked with a company to develop a pet food containing L-cysteine, which was launched on the market in 2022. It is a pet food for cats in a presymptomatic state, which, if fed to the cat daily, regularly clears small buildups of waste from the kidneys. As stated above, we have also reached the stage where we will closely be able to start the clinical trial for an injectable AIM drug.
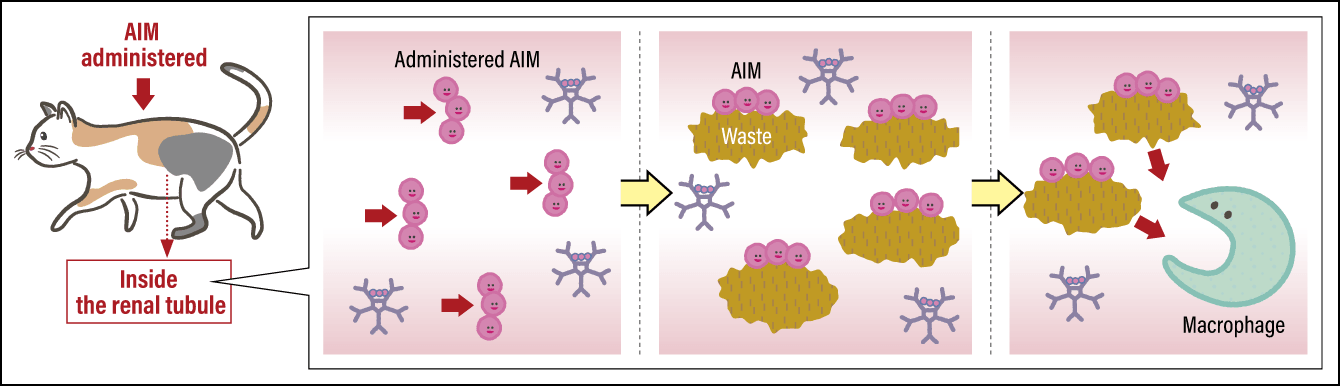
Figure 3. Mechanism of treating feline kidney diseaseWhen AIM is injected as a treatment for cats in which kidney disease has progressed, the administered AIM bonds with waste in the urine. Attracted by this marker, macrophages clear the waste by eating it. This remedies the blockage of renal tubules by waste, thereby improving kidney function.
It is not only cats in which most individuals die of kidney disease due to AIM not functioning properly. The same also applied to big cats, such as lions, cheetahs, leopards, and panthers. Cheetahs in particular have a short life expectancy, with most dying of kidney failure after around eight years, so today there are concerns that they might become extinct. It is hoped that AIM might also extend the life expectancy of such big cats and thereby save them from the risk of extinction. Of course, a drug using AIM is also being developed to treat kidney disease in humans, with efforts currently progressing about a year behind those for a feline drug.
An increase in activated AIM is a sign of disease
We have also determined that AIM’s function relates to the clearance not only of waste in the kidneys, but also of various different kinds of waste from all kinds of organs in the body. Today, the development of drugs to treat kidney disease is progressing, and it is hoped that, if these are perfected and approved, it will open the door to treatments for fatty liver, liver cancer, rheumatoid arthritis, urinary stones, and numerous neurological diseases.
It has already been demonstrated that injecting AIM into mice in which stroke has been caused dramatically reduces the mortality rate, because waste can be cleared from the brain; an article on this was published in 2021. Given this fact, I believe there is a possibility that these findings could also be applied to such conditions as Alzheimer’s disease and Parkinson’s disease, which are triggered by the chronic accumulation of waste. However, the brain is protected by what is called the blood-brain barrier, to guard against its infiltration by assorted proteins. Accordingly, the question that remains is how to cross this barrier to deliver AIM to the brain.
A method of measuring the amount of AIM in the blood has also been established. When we used this method to conduct a survey of around 20,000 people who had visited a hospital in Nagasaki Prefecture for a medical checkup, we found clear differences by gender and age, with women aged between 10 and 29 having the largest amount of AIM in their blood. The amount gradually declines as people age, with barely any difference between men and women by the time menopause begins. There might be some relationship to estrogen and other female hormones, but light has not yet been shed on this.
When a person is in a healthy state, AIM is bonded to IgM, and is activated by waste building up in the body. To put it another way, the presence of a large amount of activated AIM is a sign that some kind of disease has begun to develop. For the last year, I have been undertaking a joint study with the aforementioned Nagasaki hospital, based on the idea that, by measuring the amount of activated AIM, we might be able to achieve the early diagnosis of diseases. As we use blood collected in the course of ordinary health checkups, it does not impose an excessive burden on the test subjects. If it becomes possible to achieve the early diagnosis of diseases by measuring AIM during ordinary health checkups, we can expect to see a rise in disease treatment rates. Hopes are mounting that AIM can be skillfully used to prolong healthy life.