The potato (Solanum tuberosum) contains toxins called solanine and chaconine in its sprouts and in its skin, once it turns green. Accordingly, producers and distributors are faced with a heavy burden, due to the rigorous management required after harvesting, keeping them in a cool, dark place and preventing them from sprouting. However, the essential gene encoding an enzyme which produces these toxins have been discovered in recent years and toxin production has been successfully curbed by using genome editing to disrupt the gene. The goal for the time being is to use genome editing to deliver safe potatoes to people’s dining tables.
Special Feature 1 – An Introduction to Genome Editing Disrupting a gene to produce a “low-toxin” potato
composition by Yumi Ohuchi
It is common knowledge that we should not eat potato sprouts, because they are poisonous. While in small amounts they merely taste acrid or otherwise unpleasant, eating larger amounts causes nausea, vomiting, diarrhea, and other symptoms of food poisoning. In fact, although they might not cause serious illness or death, multiple cases of food poisoning caused by potatoes are reported in Japan alone every year. Last year, one elementary school suffered an outbreak of food poisoning when students boiled and ate potatoes that they had grown. When I examined the potatoes used, I found that they had many green patches on the skin, where toxins can accumulate as well. Although the students might have removed the sprouts, they probably did not pay attention to the skin. What makes things worse is that the toxins in potatoes increase when exposed to sunlight. These potatoes appeared to have been cultivated in a place where the soil was very shallow and were therefore exposed to sunlight.
Because potatoes have these toxins, they need to be managed rigorously after harvesting: they should be kept in a cool, dark place and treated with ethylene or the like to curb sprouting. This requirement imposes a heavy burden on producers and distributors. Moreover, to increase the safety of potatoes, processing companies have to cut away a lot of the potato when peeling and removing the sprouts from them, leading to food loss. The longest that potatoes can be preserved is a year. As the harvest in Hokkaido—Japan’s main potato-growing region—is limited in duration, disasters causing crop failure result in a lack of potatoes in storage, which also has a major impact on distribution. A lack of potatoes in 2017 led to a shortage of potato chips, which became a social issue.
Potatoes are about the only crops with toxins
Thinking about it, potatoes are about the only major crop requiring us to be cautious about toxins. Tomatoes are a close relative of potatoes, as both belong to the genus Solanum in the nightshade (Solanaceae) family. Like potatoes, tomatoes also have a toxin, which is called tomatine. However, the ripe tomato fruit that we eat today does not contain that toxin. Since ancient times, we humans have selected and cultivated not only tomatoes, but also other plants that are useful to us from among those that have mutated naturally, thereby making wild plants easier to cultivate and transforming them into larger, tastier crops.
These mutations occur when part of the DNA, which holds genetic information, is disrupted. DNA consists of two chains arranged in a double helix structure. Those chains are often damaged by ultraviolet (UV) rays and other environmental factors, but organisms have the capacity to repair them to their previous form. When humans are exposed to UV rays, the DNA chain breaks, but that does not mean that we develop a disease. However, in very rare cases, an error occurs in the repair process and a DNA sequence that differs from the original one forms, causing the organism’s properties to change. This is a spontaneous mutation. Other gene mutations can occur due to errors in copying DNA during cell division. In recent years, selective breeding—the process of artificially inducing spontaneous mutations and selecting useful varieties—has been carried out by exposing plants to radiation or ion beams (a bundle of high-energy ions).
In their wild form, potatoes were small and not very tasty, but they have been improved into the potatoes that we know today. However, humans were unable to create toxin-free potatoes. This is because, whereas humans are diploid organisms, with two sets of genomes from their two parents, potatoes are tetraploid. In other words, a potato’s cells contain four genes with the same function, so a spontaneous mutation would require all four genes to change, which is highly unlikely to occur as a natural phenomenon.
Our team —— Osaka University, RIKEN (the Institute of Physical and Chemical Research), Kobe University and National Agriculture and Food Research Organization —— is researching the creation of a toxin-free potato by using genome editing to target and disrupt specific DNA. The toxins in potatoes are solanine and chaconine, both of which are examples of compounds called steroidal glycoalkaloids (SGAs). As plants cannot move, their survival strategy involves producing diverse chemicals that are produced by the reaction of various enzymes in the course of metabolic processes. SGAs are one of such chemicals; while we knew that they were made from cholesterol, the precise mechanism of biosynthesis was unknown. Cholesterol is a lipid component also found in the human body, but whereas plants usually have very little of it, nightshades such as potatoes and tomatoes contain a lot. The reason for this was a mystery.
We explored the genes encoding enzymes of potatoes and analyzed their functions, with the result that we discovered sterol side chain reductase 2 (SSR2), a gene for an enzyme that holds the key to produce cholesterol and SGAs. In other words, if we could disrupt the SSR2 gene, we would be able to curb the production of SGAs (Figure 1).
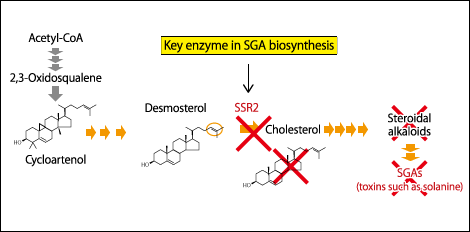
Figure 1. Snipping the SSR2 gene in a potato’s metabolic pathwayThe SSR2 enzyme responsible for biosynthesis of SGAs such as solanine and chaconine, which are toxins, is disrupted to create a potato that cannot produce SGAs.
Accordingly, we attempted to disrupt the SSR2 gene using a genome editing tool, namely transcription activator-like effector nucleases (TALEN). TALEN is an enzyme called an artificial nuclease, which, to put it in very simple terms, serves as a pair of scissors that targets and disrupt a specific gene. We introduced the TALEN gene designed to disrupt the SSR2 gene into a potato nuclear genome, using tuber slices and stems as starting material. We then carried out phytohormone treatment and cultured the seedlings. The potato that we used for our study is a cultivar called “Sassy”. As a result, we became the first in the world to successfully use genome editing to produce a potato with a DNA mutation in which the SSR2 gene was disrupted. In fact, when we investigated the content of SGAs, we found that the amount of both solanine and chaconine was much lower than in non-genome-edited potatoes (Figure 2). The content of SGAs remaining is not enough to affect the human body. This potato hardly accumulates any SGAs, even when it sprouts or turns green.
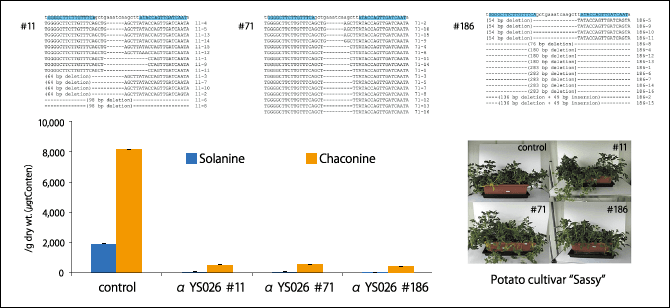
Figure 2. SGA content of potatoes whose SSR2 gene has been snipped to cause a DNA mutationThe control is a non-genome-edited potato. The content of SGAs is much lower in the genome-edited potatoes.
It is because of the presence of the sterol side chain reductase 1 (SSR1) gene that the content of SGAs does not fall to zero. The SSR1 gene is present in all plant species and is related to the biosynthesis of phytosterols (such as campesterol and sitosterol). Phytosterols are essential to the formation of the cell membrane in plants and have been applied in food for specified health uses (FOSHU) in recent years, as an ingredient that controls cholesterol in the human body. In fact, the SSR2 gene developes when the SSR1 gene duplicates and evolves to specialize in the biosynthesis of cholesterol. In our research, we know that the SSR1 gene is also involved in the biosynthesis of cholesterol, albeit only slightly, and that is why a small amount of SGAs is produced.
Regulations do not apply in the absence of exogenous genes
We use an artificial climate incubator (a device that enables temperature, sunlight, and other factors to be controlled) to culture the genome-edited potatoes. While our code of research ethics means that we cannot check the flavor at this stage, their appearance is no different from conventional potatoes (Figure 3). However, the “scissors” (TALEN) gene (hereinafter “scissors gene”) that we introduced remains in the potato’s genome, so there is a possibility that it could have some kind of adverse effect. Under the rules for genome-edited foods and foods produced by recombinant-DNA techniques published by the Consumer Affairs Agency in September 2019, foods into which exogenous genes have been implanted by means of genome editing are subject to the same regulations as foods produced by recombinant-DNA techniques, including labeling requirements and safety reviews. On the other hand, the agency issued a guideline stating that genome-edited foods that have merely had their DNA cut, without the implantation of an exogenous gene, are not subject to regulation (voluntary notification and labeling), just like conventional selectively bred foods. Naturally, we believe that safety must be guaranteed and that voluntary labeling is required, but developing genome-edited foods that are not subject to regulation is vital in order to enable them to become prevalent.

Figure 3. A genome-edited potatoWhile it is on the small side, because it was grown in a test pot, it is no different in appearance from an ordinary potato.
We are currently moving forward with research aimed at producing a toxin-free potato in which the scissors gene does not remain. Thinking about it in general terms, Mendel’s laws mean that there is some probability of producing a potato that contains neither the scissors gene nor the SSR2 gene if we cross-breed the seeds of genome-edited potatoes and nongenome-edited ones. However, seed-propagated potatoes do not breed well, so tubers rather than seeds are the primary means of growing more potatoes. In addition, seed-propagated varieties have a different genotype from their parents and have to breed afresh as a new variety. To achieve commercialization without delay, it is necessary to create a low-toxin potato using an existing variety, such as “May Queen” or “Toya”, which are particularly prone to developing high levels of SGAs.
We have focused on the properties of an Agrobacterium used in genome editing. Agrobacterium is a genus of soil bacteria into which we introduced the scissors gene. Infecting the plant with this bacterium enables the scissors gene to enter the plant’s cells. This technique is also used in the aforementioned research.
Once we infect slices of potato stem with this bacteria, many scissors form in just a few days. At this stage, the scissors gene has not yet been incorporated into the potato’s genome. Then, at a point when we surmise that the SSR2 gene has been cut, but the scissors gene has not been integrated into the plant genome, we collect seedlings obtained from the stem by means of phytohormone treatment. When we examined whether or not the seedlings contained the scissors gene, we found that in 1 case in 100, the SSR2 gene had been disrupted, the seedling did not contain the scissors gene, and the amount of both solanine and chaconine was extremely low.
We are currently engaged in research using CRISPR-Cas, which makes genome editing simpler than TALEN. In a test tube, we can produce microtubers that will form seed potatoes, and we can also produce many microtubers in special bags filled with culture medium, so we hope that this will lead to the mass production of excellent potatoes (Figure 4).

Figure 4. Microtubers and the artificial climate incubatorMicrotubers have formed on the roots. They are cultured in an artificial climate incubator (right photo), which is kept at a temperature of 20°C.
Information must be as open as possible
We also found that potatoes in which the activity of Gene A (tentative name) is curbed, do not form sprouts after harvesting, but do form them when buried in soil. Gene A is located further downstream of SSR2 in the SGA biosynthetic pathway. For producers and other related business operators, toxin-free potatoes that do not sprout would be ideal, as creating potatoes of this nature would substantially cut storage and other management costs. We plan to move forward with research aimed at bringing this phenomenon to fruition by means of genome editing. In addition, we discovered that disrupting a Gene B (tentative name), which is also located further downstream of SSR2, causes yamogenin to build up. As yamogenin is believed to be effective in preventing hyperlipidemia, this is another topic that we plan to research going forward.
Our goal for the time being is to use genome editing to deliver low-toxin potatoes to people’s dining tables. By reducing management costs, it would be possible to not only ship potatoes to other parts of Japan, but also export them to other countries. But first we need to actually cultivate these low-toxin potatoes in fields to see whether they are affected by insects or moles and what their flavor is like. We are making preparations for outdoor tests to this end, but unfortunately we have experienced delays due to the impact of the COVID-19 pandemic.
Research aimed at using genome editing technology to develop crop varieties and breeding materials is progressing in Japan, focusing not only on potatoes, but also on tomatoes with a high GABA content, wheat resistant to fusarium ear blight, seedless bell peppers, and low-allergen crops. However, the fact remains that consumers are anxious about genetic technologies. I believe that we must make information as open as possible. Our research team launched the New Potato Technology Liaison Council in 2018. We plan to work on the social implementation and expansion of our research output in partnership with researchers, producers, distributors, processors, and consumers.
Plants are estimated to produce 1 million types of chemicals. Some of these—not only those contained in potatoes—are chemicals that can be toxic to humans, but which can also become medicines. We have not yet shed light on all of these and, as a researcher, I am constantly astonished by the power of plants. Now that we can make effective use of this power through advances in genome editing technology, I believe that it is vital for us to proceed carefully, so that we can put them into practice appropriately for society and the environment.